Introduction
Traumatic brain injuries (TBIs) are a major source of morbidity and mortality, as TBIs contribute to approximately 30% of all injury-related deaths.1 A number of different mechanisms can lead to TBIs: closed head injuries, blast-related injuries, and penetrating injuries.2
TBI severity is initially classified using a Glasgow Coma Score (GSC), which has a maximum score of 15 and assesses eye opening, verbal responses, and motor responses in patients. When scoring TBI severity, a mild TBI is classified as GCS 13-15, moderate as 9-12, and severe as 3-8.1 TBIs are a heterogeneous group of injuries that cannot be differentiated necessarily by GCS alone because GCS scaling does not provide information about the specific patho-physiologic mechanisms responsible for neurological deficits.3
As such, hematomas (epidural, subdural, subarachnoid, intraparenchymal), contusions, and diffuse axonal injury all could present as severe TBIs based on the GCS classification. The terms “concussion” and “TBI” often are used interchangeably, though a concussion is a subset of TBI. Concussions are defined as low-velocity injuries that cause brain “shaking,” resulting in clinical symptoms not necessarily related to pathological injury.4,5 For the purposes of this manuscript, we will use the term TBI except in referring to the title of a concussion clinic or citing a study that specifically refers to sports-related concussions.
The majority of pediatric patients tend to recover from a concussion within 7-10 days,4,6 although recovery can be prolonged. Symptoms of minor TBI (mTBI) are often defined as physical, emotional, cognitive, and sleep. Studies have found that 11-17% of children with a mTBI remain symptomatic three months after injury.7,8 Multiple factors have been found to influence pediat-ric mTBI recovery. Younger age,9-11 female gender,12-17 past medical history of TBI,15,16,18 mood disorders,11,14,15,19,20 and sleep disturbances6,21-23 have all been associated with prolonged recovery time. Greater acute injury severity, as measured by initial symptom burden, loss of consciousness, and GCS < 15, also have been shown to be associated with more severe or longer-lasting symptoms.16,19,24-28,32 Few studies have specifically looked at recovery time in children presenting with mTBI to a pediatric trauma service, so the goal of this study was to examine factors that could influence recovery time in those patients.
Table 1. Mechanism of Injury and Recovery Time
*MVA = Motor vehicle accident
|
Number of Patients |
Median Recovery Time (Days) |
P Value (vs. MVA) |
P Value (vs. Fall) |
|
|
Sport/Recreation |
54 (41%) |
33 |
0.962 |
0.0586 |
|
MVA* |
44 (33%) |
31 |
- |
0.135 |
|
Fall |
31 (23%) |
39.5 |
0.135 |
- |
|
Assault/Child Abuse |
3 (2%) |
31 |
- |
- |
|
Firearm |
1 (1%) |
143 |
- |
- |
Table 2. GCS and ICH
*ICH = Intercranial hemorrhage
|
Number of Patients |
Median Recovery Time (Days) |
|||||
|
GCS 3-12 |
GCS 13-15 |
GCS Unknown |
GCS 3-12 |
GCS 13-15 |
GCS Unknown |
|
|
GCS, n(%) |
17 (13%) |
99 (74%) |
17 (13%) |
34 |
32 |
0.393 |
|
No ICH |
ICH |
No ICH |
ICH |
P Value |
||
|
ICH,* n(%) |
77 (58%) |
55 (41%) |
30 |
34 |
0.506 |
|
Methods
Data Collection
A retrospective chart review was conducted on patients admitted for TBI to a pediatric trauma service at a Level 1 pediatric trauma center between January 1, 2014 and December 31, 2015. Patients within this 2-year time period were included in the study if they met the following inclusion criteria: aged 5-17, discharged to home, and a discharge diagnosis of TBI, concussion, intracranial hemorrhage (ICH), or mention of a TBI diagnosis in the patient’s history and physical. The age range was chosen to analyze school-aged children. ICH included all hemorrhages — epidural, subdural, subarachnoid, intraparenchymal and contusion — identified on CT scan. Patients were excluded if they experienced an additional TBI before recovering from the initial injury. The selected patients were identified by filtering the trauma database for ICD 9 codes 850-854 (intracranial injury, excluding those with skull fracture). ICD 9 codes 800-804 also were checked to see if inclusion of skull fracture would increase the number of patients with TBIs, but none were found. Patients who had a TBI as well as an additional extracranial injury were not excluded. Stratification of the trauma data identified a total of 169 patients, of which 36 patients did not have a discharge diagnosis of TBI, concussion, or ICH and thus were excluded. Of the 133 patients who met the final inclusion criteria, 94 had definitive recovery time data available. Median recovery time was reported.
Using the information contained in the inpatient and outpatient electronic medical records, data was recorded regarding patient demographics, past medical history, mechanism of injury, injury presentation (GCS, ICH, presenting symptoms), inpatient treatment and length of stay, time missed from school, outpatient follow-up and treatment, longitudinal treatment and symptoms (sleep, mood, cognition, headache), and recovery time. Mechanism of injury was separated into sports/recreation, motor vehicle accident, fall-related injury, assault/child abuse, and firearm-related injury. Due to a lack of considerably depressed GCS scores, GCS was grouped into patients with severely or moderately depressed scores (GCS 3-12) and mildly depressed scores (GCS 13-15).
Recovery time was established as the length of time from the date of initial injury to date at which a provider note was written with one of the following: the patient was asymptomatic; follow up only as needed; or patient did not need to return for greater than six months. Recovery time was considered to be unknown if the patient did not attend a follow-up appointment or scheduled a follow-up appointment at another institution. The study was reviewed and approved by the Institutional Review Board (IRB).
Statistical analysis
Exploratory analyses were performed using R 3.4.1 (R Core Team),29 including the computation of summary statistics and the creation of contingency tables. Boxplots were made to compare distributions of recovery time in groups defined by select categorical clinical variables.
Table 3. Past Medical History
ADHD = Attention-Deficit/Hyperactivity Disorder, PMH = Past Medical History, TBI = Traumatic Brain Injury
|
Number of Patients
|
Median Recovery Time
|
|||||||||
|
Anxiety and/or depression, n (%) |
128 (96%) |
5 (4%) |
32 |
143 |
0.009 |
|||||
|
Autism, n (%) |
128 (96%) |
5 (4%) |
33 |
38.5 |
0.691 |
|||||
|
ADHD, n (%) |
116 (87%) |
17 (13%) |
32 |
45 |
0.0119 |
|||||
|
Learning disability, n (%) |
132 (99%) |
1 (1%) |
33 |
13 |
0.162 |
|||||
|
Migraine, n (%) |
126 (95%) |
7 (5%) |
33 |
37 |
0.772 |
|||||
|
Seizure disorder, n (%) |
129 (97%) |
4 (3%) |
33 |
24.5 |
0.427 |
|||||
|
Previous TBI, n (%) |
124 (93%) |
9 (7%) |
33 |
39 |
0.428 |
|||||
Results were reviewed manually to select factors to be analyzed. Wilcoxon signed rank tests were applied to compare recovery times to fixed reference values identified in previous studies, whereas Wilcoxon rank sum tests were used to compare recover times between two groups of patients defined by categorical variables of in-terest. All tests were performed at the α = 0,05 level, and a Bonferroni adjustment was used to account for multiple testing. Correlation-based analysis was not appropriate in this study because analysis was not conducted involving two quantitative variables
Results
The average age of patients in this study was 10.8 years. Sixty-three percent of patients were male (n = 84) and had a median recovery time of 32.5 days, whereas females had a median recovery time of 34.0 days. Recovery times were not significantly different between the genders (p = 0.373). The median recovery time for all patients was 33.0 days.
Table 1 shows mechanism of injury and median recovery time. Forty-one percent of patients suffered from a sports/recreation-related injury (n = 54), 33% of patients were involved in a motor vehicle accident (n = 44), and 23% of patients were involved in a fall-related injury (n = 31). A comparison of mechanism of injury and recovery time showed recovery was not significantly different between sport-related, motor vehicle accidents, and fall-related injuries. Not enough assault or firearm victims were included in this study to be studied.
Initial GCS and ICH were not associated with median recovery time (Table 2). Thirteen percent of patients (n = 17) presented with a significantly or moderately depressed GCS 3-12 and had a median recovery rate of 34.0 days, whereas 74% of patients (n = 99) presented with a mildly depressed GCS 13-15 and had a median recovery time of 32.0 days (p = 0.393). Patients with no ICH had a median recovery of 30.0 days, whereas those with ICH had a median recovery of 34.0 days (p = 0.506).
Past medical history also was analyzed as a factor that could affect median recovery time (Table 3). Four percent of patients (n = 5) had a positive PMH of anxiety and/or depression, which was associated with a longer median recovery time (143.0 days) compared to a negative PMH of anxiety and/or depression (32.0 days) (p = 0.009). Patients with ADHD also had a longer recovery time (45.0 days) than patients without (32.0 days) (p = 0.012). Patients with autism, learning disabilities, migraines, or previous TBI each had longer median recovery times than the patients with negative PMHs, but none of these comparisons reached statistical significance.
Longitudinal complications of TBI were analyzed in relationship to median recovery time (Table 4). Thirteen percent of patients (n = 17) complained of sleep disturbances (difficulty falling asleep, staying asleep, or both) at one of their follow-up appointments and had a median recovery time of 53.5 days that was significantly longer than patients without a sleep disturbance (p = 0.002). Seventeen percent of patients with cognitive complications, such as difficulty concentrating, impaired memory, or slower processing speed, had a longer recovery than patients with no change from baseline (p = 0.035). However, using Bonferroni correction, P must be less than 0.0085 to be significant. Therefore, the increased recovery time with cognitive longitudinal complications is no longer significant. Symptoms of mood, headache, balance, and dizziness also showed increased median recovery times but these were not statistically different from the overall median recovery of 33.0 days.
Patients in the current study were admitted to an inpatient, pediatric trauma service. In contrast, most of the previous literature involves patients who are seen in an outpatient setting. Thomas et al. (2018) attempted to determine a precise pediatric recovery rate for those seen in outpatient clinics.16 Patients aged 10-17 (n=1733) with sports-related concussions presented to seven concussion clinics and were found to have a median recovery of 17 days. Our inpatient study consisted of patients with significantly longer recovery times (33 days). Both patients with a severely or moderately depressed GCS 3-12 had a longer median recovery time than 17 days (34 days, p = 0.002) as did patients with a mildly depressed GCS 13-15 (32 days, p ≤ 0.0001) (Figure 1).
Table 4. Longitudinal Symptoms Following TBI
|
Number of Patients |
Median Recovery Time (Days) |
|||||
|
No Change |
Symptomatic |
Unknown |
No Change |
Symptomatic |
No Change vs. Symptomatic |
|
|
Sleep |
102 (77%) |
17 (13%) |
14 (11%) |
32 |
53.5 |
0.002 |
|
Mood |
101 (76%) |
18 (14%) |
14 (11%) |
32 |
41 |
0.226 |
|
Cognition |
97 (73%) |
22 (17%) |
14 (11%) |
32 |
46 |
0.036 |
|
Headache |
72 (54%) |
47 (35.3%) |
14 (11%) |
32 |
36 |
0.198 |
|
Balance |
112 (84%) |
7 (5%) |
14 (11%) |
32.5 |
53 |
0.124 |
|
Dizziness |
102 (77%) |
17 (13%) |
14 (11%) |
32 |
43 |
0.099 |
Discussion
Multiple factors previously have been shown to influence recovery from a TBI.9-28,32 In this retrospective chart review study, we found that patients with post-concussive sleep disturbances and comorbid mood disorders showed an increased median recovery length. Moreover, the overall median recovery time for patients admitted to the trauma service was longer compared to outpatient studies. These results may inform clinicians regarding patients who may experience longer recovery times.
The relationship between gender and TBI recovery time has been well documented. Prior studies have demonstrated that females have a prolonged recovery time compared to males.13-16 The current study did not show the same association. The cause of the lack of a difference is unclear. This is an interesting finding that could be related to the particular patient population because most other studies show prolonged female recovery in outpatient, sports-related concussions, but more research should be conducted to further explore this question.
This study showed a significantly longer median recovery time than Thomas et. al found in its outpatient study.16 This longer median recovery time could suggest that patients admitted to a pediatric trauma service are at risk for prolonged recovery. However, it should be noted that these studies cannot be directly compared, as Thomas et. al, focused on sports-related concussions, which is a only subset of the mTBI patients included in this study.
The GCS is a common assessment tool utilized after head injury. Whereas some studies suggest that its initial severity, as defined by degree of symptoms, can predict TBI recovery,16,19,24-28,32 the current study found no association between initial GCS score and recovery time. This could have been due to GCS not being a good predictor of prolonged recovery with less severe TBI.32,33 Another consideration is that all patients included in this study improved to the point they could be discharged home. Therefore, patients with a low initial GCS who had a more severe initial presentation and might have been at risk for a longer recovery could have been excluded from this study because they were instead discharged to a rehabilitation setting.
The relationship between GCS and intracranial hemorrhage (ICH) is unclear, as some studies suggest an inverse relationship while others suggest no correlation.34,35 However, ICH can be used to assess injury severity, as it can lead to worse, potentially life-threatening, patient outcomes and is thought to be an important criteria in classifying TBI by pathology.33 ICH has been shown to be associated with prolonged recovery from increased cognitive symptoms and functional impairment.36-38 In this study, the presence of ICH was not a factor in recovery time. One possible explanation is that the presence of ICH was not a prognostic factor because the lesions were too small to prolong recovery. Another explanation is that while ICH may be associated with increased initial symptom burden, it may not overly prolong recovery. One study found that patients with intracranial pathology have worse quality of life at three months, but the adverse consequences resolved before one year.36 Collectively, the data suggest that the use of either the GCS or presence of ICH is equivocal for predicting recovery after TBI. Although GCS and ICH were not shown to be associated with recovery time in our study, the prolonged recovery time of patients in this study compared to prior studies may reflect more severe injury that prompted an inpatient admission for these patients.
This analysis supports previous studies that demonstrate an association between premorbid psychiatric conditions and prolonged recovery time following TBI, though our sample size for these conditions was quite low (n=5). Each patient with anxiety or depression, however, had a longer recovery time than the overall median recovery time of 33.0 days (34, 49, 143, 434, and 435 days). One potential confounding factor could be the linkage between anxiety, depression, and poor sleep quality. In general, anxiety and depression are associated with poor sleep quality.30 After TBI, post-concussive depression has been shown to predict the presence of insomnia.31 The relationship between poor sleep, anxiety and depression, however, does not explain the risk of prolonged recovery in patients with anxiety or depression who do not have noticeable sleep difficulties.11,14,15,19,20 Along with anxiety and depression, ADHD also was found to increase recovery time (n = 17). All patients in this group had a longer recovery time than the overall median recovery time of 32.0 days. These findings support the need for proper psychiatric screening of patients presenting with TBI.
Patients with sleep disturbance had a prolonged recovery as compared to patients without. Current literature shows that sleep disturbance, such as increased sleep need, daytime sleepiness, or insomnia aggravate other sequelae of TBI and significantly prolong recovery from TBI.6,21,22 Sleep disturbance has been found to be associated with a three- to fourfold increase in pediatric recovery time and may not present immediately after injury.6,23 Thus, it is important not only to identify these patients on initial evaluation, but to educate patients to be aware of the possibility of sleep disturbances and the need to follow up in a concussion clinic if these symptoms arise.
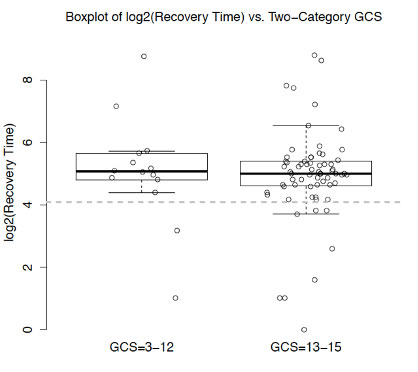
Figure 1. Comparison of severely and mildly depressed GCS to 17-day recovery time |
Limitations
Several potential limitations should be noted. The data may not represent patient populations from different regions of the world. Only 79% of patients (n = 133) met all of the inclusion criteria for this study and this high exclusion rate could have prevented certain statistical comparisons from becoming significant. Twenty-nine percent of patients (n = 39) had unknown recovery times, either due to follow up out of network or because they did not attend scheduled follow-up visits. Recovery was measured as the length of time from the initial injury to the last day of follow-up. Recovery time also could have been overestimated in this study if a large number of patients with unknown follow up did not return because they were already asymptomatic. This value also could have been overestimated by patients’ last visit occurring after full recovery, as well as delayed recovery from other traumatic injuries, such as skull fracture. In contrast, recovery could have been underestimated by patients having activity restrictions or mild to moderate symptoms on the last day of follow up. When calculating recovery time, we attempted to differentiate recovery due to TBI from other inju-ries. When looking at follow-up notes, patients were only considered recovering if symptoms were noted to be due to TBI and not extracranial injuries. This is an imperfect solution and extracranial injuries still influenced recovery time.
GCS was obtained from a variety of sources such as outside hospitals or the EMS, arrival at our institution may have been obtained at different times post injury, and categorical values were not obtained. To overcome this obstacle in the future, a uniform time point should be cho-sen to obtain GCS, such as presentation in the emergency department. The location of ICH was recorded, but the volume of hematoma or presence of midline shift was not. It is possible that the patients in this study who were ICH positive had small hemorrhages not significant enough to delay recovery. Because not all patients underwent imaging, it is also possible that patients with undiagnosed ICH were included in this study. Finally, this was a retrospective chart review and not all data were available for each patient. Whereas all of the trauma providers were experts in TBI, it is possible that each medical discipline (pediatrics, etc.) does not each equally address specific information that was recorded in this study. In particular, past medical history such as behavioral health disorders, most likely was underdiagnosed.
Conclusion
This study found that GCS score and ICH upon admission are not prognostic factors for TBI recovery, whereas sleep and behavioral health are associated with prolonged TBI recovery. Patients admitted to a pediatric trauma service may be at risk for prolonged recovery. Therefore, these centers need to assess for sleep and be-havioral health disorders prior to discharge and provide anticipatory guidance for follow up. A well-designed prospective study should be conducted to confirm our findings and provide additional guidelines.
Author Information
Corresponding Author
*Andrew Feldman, MD, MEd,
anf9170@nyp.org
Author was a Penn State College of Medicine student at time of submission.
Author Contributions
All authors have given approval to the final version of the manuscript.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Disclosures
No authors have any disclosures or conflicts of interest at this time.
Acknowledgements
We express gratitude for the generous support of Penn State College of Medicine and its staff who assisted with IRB approval. Vonn Walter, PhD, was instrumental in assisting in data analysis. We would also like to thank the Department of Pediatric Surgery, who sponsored attendance at the 2019 Child Neurology Society Annual Meeting.
References
1. Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic Brain Injury–Related Emergency Department Visits, Hospitalizations, and Deaths — United States, 2007 and 2013. MMWR Surveill Summ. 2017;66(9):1-16. doi:10.15585/mmwr.ss6609a1
2. Bauer D, Tung ML, Tsao JW. Mechanisms of Traumatic Brain Injury. Semin Neurol. 2015;35(1):e17-e22. doi:10.1055/s-0035-1549095
3. Saatman KE, Duhaime A-C, Bullock R, Maas AIR, Valadka A, Manley GT. Classification of traumatic brain injury for targeted therapies. J Neurotrauma. 2008;25(7):719-738. doi:10.1089/neu.2008.0586
4. Mccrory P, Meeuwisse WH, Aubry M, Cantu RC, Dvořák J, Echemendia RJ, Engebretsen L, Johnston K, Kutcher JS, Raftery M, Sills A, Benson BW, Davis GA, Ellenbogen R, Guskiewicz KM, Herring SA, Iverson GL, Jordan BD, Kissick J, Mccrea M, Mcintosh AS, Maddocks D, Makdissi M, Purcell L, Putukian M, Schneider K, Tator CH, Turner M. Consensus Statement on Concussion in Sport: The 4th International Conference on Concussion in Sport, Zurich, November 2012. Journal of Athletic Training. 2013;48(4):554-575. doi:10.4085/1062-6050-48.4.05.
5. McCrory P, Meeuwisse W, Dvorak J, Aubry M, Bailes J, Broglio S, Cantu RC, Cassidy D, Echemendia RJ, Castellani RJ, Davis GA, Ellenbogen R, Emery C, Engebretsen L, Feddermann-Demont N, Giza CC, Guskiewicz KM, Herring S, Iverson GL, Johnston KM, Kissick J, Kutcher J, Leddy JJ, Maddocks D, Makdissi M, Manley GT, McCrea M, Meehan WP, Nagahiro S, Patricios J, Putukian M, Schneider KJ, Sills A, Tator CH, Turner M, Vos PE. Consensus statement on concussion in sport—the 5th international conference on concussion in sport held in Berlin, October 2016. British Journal of Sports Medicine . 2017;51:838-847.
6. Bramley H, Henson A, Lewis MM, Kong L, Stetter C, Silvis M. Sleep Disturbance Following Concussion Is a Risk Factor for a Prolonged Recovery. Clin Pediatr (Phila). 2017;56(14):1280-1285. doi:10.1177/0009922816681603
7. Barlow KM, Crawford S, Stevenson A, Sandhu SS, Belanger F, Dewey D. Epidemiology of postconcussion syndrome in pediatric mild traumatic brain injury. Pediatrics. 2010;126(2):e374-e381. doi:10.1542/peds.2009-0925
8. Ponsford J, Willmott C, Rothwell A, Cameron P, Ayton G, Nelms R, Curran C, Ng K. Cognitive and Behavioral Outcome Following Mild Traumatic Head Injury in Children . Journal of Head Trauma Rehabilitation. 1999;14(4):360-372.
9. Field M, Collins MW, Lovell MR. Does age play a role in recovery from sports related concussions? A comparison of high school and collegiate athletes. . Am J Pediatr. 2003;142:546-553. doi:10.1067/mpd.2003.190
10 Pellman EJ, Lovell MR, Viano DC, Casson IR. Concussion in professional football: Recovery of NFL and high school athletes assessed by computerized neuropsychological testing - Part 12. Neurosurgery. 2006;58(2):263-272. doi:10.1227/01.NEU.0000200272.56192.62
11. Corwin DJ, Grady MF, Joffe MD, Zonfrillo MR. Pediatric Mild Traumatic Brain Injury in the Acute Setting. Pediatr Emerg Care. 2017;33(9):643-649. doi:10.1097/PEC.0000000000001252
12. Dick RW. Is there a gender difference in concussion incidence and outcomes? In: British Journal of Sports Medicine. Vol 43. ; 2009. doi:10.1136/bjsm.2009.058172
13. Bramley H, Heverley S, Lewis MM, Kong L, Rivera R, Silvis M. Demographics and Treatment of Adolescent Posttraumatic Headache in a Regional Concussion Clinic. Pediatr Neurol. 2015;52(5):493-498. doi:10.1016/j.pediatrneurol.2015.01.008
14. Guerriero RM, Kuemmerle K, Pepin MJ, Taylor AM, Wolff R, Meehan WP. The Association Between Premorbid Conditions in School-Aged Children With Prolonged Concussion Recovery. J Child Neurol. 2018;33(2):168-173. doi:10.1177/0883073817749655
15. Miller JH, Gill C, Kuhn EN, Rocque BG, Menendez JY, Oneill JA, Agee BS, Brown ST, Crowther M, Davis RD, Ferguson D, Johnston JM. Predictors of delayed recovery following pediatric sports-related concussion: a case-control study. Journal of Neurosurgery: Pediatrics. 2016;17(4):491-496. doi:10.3171/2015.8.peds14332.
16. Thomas DJ, Coxe K, Li H, Pommering TL, Young JA, Smith GA, Yang J. Length of Recovery from Sports-Related Concussions in Pediatric Patients Treated at Concussion Clinics. Clinical Journal of Sport Medicine. 2018;28(1):56-63. doi:10.1097/jsm.0000000000000413.
17. Colvin AC, Mullen J, Lovell MR, West RV, Collins MW, Groh M. The role of concussion history and gender in recovery from soccer-related concussion. Am J Sports Med. 2009;37(9):1699-1704. doi:10.1177/0363546509332497
18. Schatz P, Moser RS, Covassin T, Karpf R. Early indicators of enduring symptoms in high school athletes with multiple previous concussions. Neurosurgery. 2011;68(6):1562-1567. doi:10.1227/NEU.0b013e31820e382e
19. Iverson GL, Gardner AJ, Terry DP, Ponsford JL, Sills AK, Broshek DK, Solomon GS. Predictors of clinical recovery from concussion: a systematic review. British Journal of Sports Medicine. 2017;51(12):941-948. doi:10.1136/bjsports-2017-097729.
20. Corwin DJ, Zonfrillo MR, Master CL, Arbogast KB, Grady MF, Robinson RL, Goodman AM, Wiebe DJ. Characteristics of Prolonged Concussion Recovery in a Pediatric Subspecialty Referral Population. The Journal of Pediatrics. 2014;165(6):1207-1215. doi:10.1016/j.jpeds.2014.08.034.
21. Ouellet MC, Beaulieu-Bonneau S, Morin CM. Sleep-wake disturbances after traumatic brain injury. Lancet Neurol. 2015;14(7):746-757. doi:10.1016/S1474-4422(15)00068-X
22. Murdaugh DL, Ono KE, Reisner A, Burns TG. Assessment of Sleep Quantity and Sleep Disturbances During Recovery From Sports-Related Concussion in Youth Athletes. Archives of Physical Medicine and Rehabilitation. 2018.
23. Eisenberg MA, Meehan WP, Mannix R. Duration and Course of Post-Concussive Symptoms. Pediatrics. 2014;133(6):999-1006. doi:10.1542/peds.2014-0158
24. Meehan WP, Mannix RC, Stracciolini A, Elbin RJ, Collins MW. Symptom severity predicts prolonged recovery after sport-related concussion, but age and amnesia do not. J Pediatr. 2013;163(3):721-725. doi:10.1016/j.jpeds.2013.03.012
25. Meehan WP, Mannix R, Monuteaux MC, Stein CJ, Bachur RG. Early symptom burden predicts recovery after sport-related concussion. Neurology. 2014;83(24):2204-2210. doi:10.1212/WNL.0000000000001073
26. Eisenberg MA, Andrea J, Meehan W, Mannix R. Time Interval Between Concussions and Symptom Duration. Pediatrics. 2013;132(1):8-17. doi:10.1542/peds.2013-0432
27. Zemek R, Barrowman N, Freedman SB, Gravel J, Gagnon I, Mcgahern C, Aglipay M, Sangha G, Boutis K, Beer D, Craig W, Burns E, Farion KJ, Mikrogianakis A, Barlow K, Dubrovsky AS, Meeuwisse W, Gioia G, Meehan WP, Beauchamp MH, Kamil Y, Grool AM, Hoshizaki B, Anderson P, Brooks BL, Yeates KO, Vassilyadi M, Klassen T, Keightley M, Richer L, Dematteo C, Osmond MH. Clinical Risk Score for Persistent Postconcussion Symptoms Among Children With Acute Concussion in the ED. Jama. 2016;315(10):1014. doi:10.1001/jama.2016.1203.
28. Brown NJ, Mannix RC, O’Brien MJ, Gostine D, Collins MW, Meehan WP. Effect of Cognitive Activity Level on Duration of Post-Concussion Symptoms. Pediatrics. 2014;133(2):e299-e304. doi:10.1542/peds.2013-2125
29. R Foundation for Statistical Computing. R: A Language and Environment for Statistical Computing. Vol 1.; 2011. http://www.r-project.org.
30. Mayers AG, Grabau EA, Campbell C, Baldwin DS. Subjective sleep, depression and anxiety: inter-relationships in a non-clinical sample. Hum Psychopharmacol. 2009;24(6):495-501. doi:10.1002/hup.1041
31. Ouellet M-C, Beaulieu-Bonneau S, Morin CM. Insomnia in Patients With Traumatic Brain Injury Frequency, Characteristics, and Risk Factors. J Head Trauma Rehabil. 2006;21(3):199-212. doi:10.1097/00001199-200605000-00001
32. Evans E, Asuzu D, Cook NE, Caruso P, Townsend E, Costine-Bartell B, Fortes-Monteiro C, Hotz G, Duhaime A-C, Ramon D, Joseph G, Geoff M, Pratik M, David O, Claudia R, Nancy T. Traumatic Brain Injury-Related Symptoms Reported by Parents: Clinical, Imaging, and Host Predictors in Children with Impairments in Consciousness Less than 24 Hours. Journal of Neurotrauma. 2018;35(19):2287-2297. doi:10.1089/neu.2017.5408.
33. Saatman KE, Duhaime A-C, Bullock R, Maas AI, Valadka A, Manley GT. Classification of Traumatic Brain Injury for Targeted Therapies. Journal of Neurotrauma.2008;25(7):719-738. doi:10.1089/neu.2008.0586.
34. Hsiang JNK, Yeung T, Yu ALM, Poon WS. High-risk mild head injury. Journal of Neurosurgery. 1997;87(2):234-238. doi:10.3171/jns.1997.87.2.0234.
35. Simon B, Letourneau P, Vitorino E, Mccall J. Pediatric Minor Head Trauma: Indications for Computed Tomographic Scanning Revisited. The Journal of Trauma: Injury, Infection, and Critical Care. 2001;51(2):231-238. doi:10.1097/00005373-200108000-00004.
36. Rivara FP, Koepsell TD, Wang J, Temkin N, Dorsch A, Vavilala MS, Durbin D, Jaffe KM. Disability 3, 12, and 24 Months After Traumatic Brain Injury Among Children and Adolescents. Pediatrics. 2011;128(5). doi:10.1542/peds.2011-0840d.
37. Levin HS, Hanten G, Roberson G, Li X, Ewing-Cobbs L, Dennis M, Chapman S, Max JE, Hunter J, Schachar R, Luerssen TG, Swank P. Prediction of cognitive sequelae based on abnormal computed tomography findings in children following mild traumatic brain injury. Journal of Neurosurgery: Pediatrics. 2008;1(6):461-470. doi:10.3171/ped/2008/1/6/461.
38. Swanson JO, Vavilala MS, Wang J, Pruthi S, Fink J, Jaffe KM, Durbin D, Koepsell T, Temkin N, Rivara FP. Association of initial CT findings with quality-of-life outcomes for traumatic brain injury in children. Pediatric Radiology. 2012;42(8):974-981. doi:10.1007/s00247-012-2372-8.