Learning Objectives
1. Recognize the pathognomonic magnetic resonance imaging (MRI) feature of manganese toxicity
2. Appreciate the possibility of Mn toxicity causing acute encephalopathy in the absence of chronic hepatic disease
3. Understand the absorption of Mn and its route into the central nervous system
Case Description
A 77-year-old female with a history of deep vein thrombosis on warfarin presented to the emergency department with confusion. She was found in her home lying on the floor and mumbling. Her past medical history includes a recent diagnosis of lower extremity fungal cellu-litis, as well as type II diabetes, COPD and asthma, without renal impairment. She is retired, lives at home alone in a suburban area, and denies tobacco or illicit drug use.
On physical exam, she was somnolent, but arousable with normal vital signs. She was oriented to person, place, and time. No paresthesias, tremors, or ataxia were noted on neurologic exam. Cardiac exam revealed irregular rate and rhythm. Abdominal exam was without hepatomegaly or ascites. Right lower extremity showed an erythematous, circumferential patch, slightly warm to touch. Laboratory studies are shown in Table 1. Electrocardiogram showed premature ventricular contractions and atrial fibrillation. Head computed tomography was negative for hemorrhage.
Without clear etiology of acute encephalopathy, the patient was treated for sepsis secondary to cellulitis with cefazolin. On further investigation, a brain MRI was obtained, revealing bilateral symmetrical T1 hyperintensities in the globus pallidus (GP; Figure 1).
Given this finding, serum heavy metals, including manganese (Mn), were ordered on hospital day 2. By hospital day 6, she clinically improved and was medically stable for discharge. Thereafter, Mn was found to be elevated at 41.4 µg/L. Subsequently, outpatient follow-up was arranged.
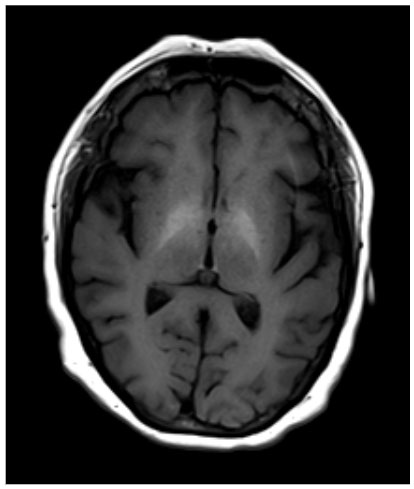
Figure 1. Brain MRI without contrast depicting bilateral symmetrical T1 hyperintensity in the globus pallidus.
Discussion
We present the first case to our knowledge of Mn toxicity resulting in acute encephalopathy in the absence of chronic liver disease and total parenteral nutrition (TPN). Mn is found in various tissues within the human body, with normal blood concentrations being 4-15 µg/L.1 While Mn is naturally found in the environment, Mn toxicity commonly results from occupational exposure in welders and smelters, contaminated water, and chronic TPN.1-3 In these high risk populations, inhaled Mn may be transported via specialized olfactory neurons into the brain, the main pathologic target of Mn toxicity.4-5 Through the gastrointestinal tract, ingested Mn may also be absorbed into the bloodstream, where it penetrates the CNS via various transporters.6 Mn is retained in peripheral tissues for 5-7 days, although retention in CNS tissue may be longer. Elimination is mediated through the fecal hepatobiliary system.1
Mn has particular predilection for the GP. Numerous case studies have implicated bilateral GP hyperintensities in Mn-exposed welders and smelters,7 leading to parkinsonism.8 Furthermore, Mn toxicity may lead to bradycardia and arrhythmias.9 Mn excretion is primarily via the hepatobiliary system.1 Impaired hepatobiliary excretion in the setting of chronic liver disease may lead to rapid Mn accumulation, which has been shown to exacerbate hepatic encephalopathy.10
In contrast to current literature, our case describes acute encephalopathy likely secondary to Mn toxicity in the absence of significant liver dysfunction. Her improvement throughout the hospital course may be due to the natural clearance of Mn, rather than response to cefazolin, considering she did not meet sepsis criteria. It is difficult to assess these points, though, given the patient’s baseline Mn level and the trend of Mn were unknown. However, with her specific brain MRI findings, cardiac presentation, and serum Mn levels, her acute encephalopathy is likely attributed to Mn toxicity. Overall, practitioners should consider encephalopathy secondary to Mn accumulation even in the absence of hepatic disease.
Table 1. Pertinent Laboratory Studies*
*WBC=white blood cell count (K/uL); Hgb=hemoglobin (g/dL); ALT=alanine aminotransferase (u/L); AST=aspartate aminotransferase (u/L); T Bili=total bilirubin; D Bili=direct bilirubin; Lactic acid (mmol/L); Alk phos=alkaline phosphatase (u/L); INR=international normalized ratio; Ace=acetaminophen; Drug Tox=drug toxicology; UA=urinalysis; Urine Cx=urine culture; Blood Cx=blood culture; H=high; L=low; Neg=negative; Bolded, red data=abnormal lab value
|
WBC |
Hgb |
ALT |
AST |
T Bili |
D Bili |
Lactic Acid |
Alk Phos |
|
7.99 |
10.1 (L) |
36 (H) |
54 (H) |
3.9 (H) |
1.2 (H) |
2.7 (H) |
108 |
|
INR |
Troponin |
Ace |
Alcohol |
Drug Tox |
UA |
Urine Cx |
Blood Cx |
|
3.4 (H) |
< 0.01 |
Neg |
Neg |
Neg |
Normal |
No growth |
No growth |
Author Information
Corresponding Author
* James V Nguyen, BS
jnguyen4@pennstatehealth.psu.edu, 717-439-8689
Author Contributions
All author(s) have given approval to the final version of the manuscript.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Disclosures
No author(s) have any disclosures or conflicts of interest at this time.
Acknowledgements
None.
References
1. O’Neal, S. L., & Zheng, W. (2015). Man-ganese toxicity upon overexposure: a decade in review. Curr Environ Health Rep, 2(3), 315-328.
2. Kondakis, X. G., Makris, N., Leotsinidis, M., Prinou, M., & Papapetropoulos, T. (1989). Possible health effects of high manganese concentration in drinking water. Archives of Environmental Health: An International Journal, 44(3), 175-178.
3. Chalela, J. A., Bonillha, L., Neyens, R., & Hays, A. (2011). Manganese encepha-lopathy: an under-recognized condition in the intensive care unit. Neurocritical Care, 14(3), 456-458.
4. Lucchini, R. G., Dorman, D. C., Elder, A., & Veronesi, B. (2012). Neurological im-pacts from inhalation of pollutants and the nose–brain connection. Neurotoxicology, 33(4), 838-841.
5. Leavens, T. L., Rao, D., Andersen, M. E., & Dorman, D. C. (2007). Evaluating transport of manganese from olfactory mucosa to striatum by pharmacokinetic modeling. Toxicological Sciences, 97(2), 265-278.
6. Yokel, R. A. (2009). Manganese flux across the blood–brain barrier. Neuromolecular Medicine, 11(4), 297-310.
7. Jiang, Y., Zheng, W., Long, L., Zhao, W., Li, X., Mo, X., ... & Long, Q. (2007). Brain magnetic resonance imaging and manganese concentrations in red blood cells of smelting workers: search for bi-omarkers of manganese exposure. Neurotoxicology, 28(1), 126-135.
8. Guilarte, T. R. (2010). Manganese and Parkinson’s disease: a critical review and new findings. Environmental Health Perspectives, 118(8), 1071-1080.
9. Jiang, Y., & Zheng, W. (2005). Cardio-vascular toxicities upon managanese exposure. Cardiovascular Toxicology, 5(4), 345-354.
10 Kobtan, A. A., El-Kalla, F. S., Soliman, H. H., Zakaria, S. S., & Goda, M. A. (2016). Higher grades and repeated recurrence of hepatic encephalopathy may be re-lated to high serum manganese levels. Biological Trace Element Research, 169(2), 153-158.