Background
The idiopathic inflammatory myopathies, including dermatomyositis (DM) and polymyositis (PM), are a group of chronic inflammatory muscle disorders characterized by progressive symmetric proximal muscle weakness. They are thought to be caused by a humoral-mediated autoimmune disease. DM, unlike PM, is associated with characteristic skin findings including Gottron’s papules, heliotrope eruption, and facial erythema. While the skin and muscle are most frequently affected, DM may also affect the joints, esophagus, and lungs.1 Studies have found that DM affects as many as 21.42 persons per 100,000 in the United States.2
DM is strongly associated with the development of malignancy. Since the association between DM and cancer was described in 1916, numerous studies have been performed to quantify the risk.3,4 According to a 2015 meta-analysis, which included publications from various countries worldwide, the relative risk of malignancy in DM patients is 5.50 (95% CI 4.31-7.70).5 The largest cohort study, which included 1,012 DM patients from Taiwan, estimated the standardized incidence ratio (SIR) for malignancy in DM patients as 5.11 (95% CI 5.01-5.22).6 Other studies estimate SIRs between 3.0 and 7.7 with malignancy occurring in 13.8 to 25% of DM patients.7-16 Although females are more likely to have DM, males are more likely to develop paraneoplastic disease.5,10,13 The risk of malignancy also increases with age.10,12,13 Other features suggested to be predictive of malignancy include the presence of cutaneous necrosis, shawl sign, dysphagia, elevated CK, LDH, AST, or ALT, and age greater than 40 in Asian populations, or 52 in Europeans.17-22
Previous studies have attempted to determine the period of greatest malignancy risk with respect to DM diagnosis. Madan et al. found that the risk is elevated three years prior to diagnosis of DM and five years after diagnosis, while András et al. found that risk is elevated two years before and three years after diagnosis.23,24 Other studies have found the greatest SIRs have been reported during the first year after the diagnosis of DM.9,10,14
The most common types of malignancy associated with DM are lung, colon, breast, and gastric.6,8,9,12,14,18 The risk for ovarian cancer is well established to be uniquely high, with reports of a seventeenfold increased risk over the rest of the population.8,25 Other malignancies associated with DM include leukemia/lymphoma, thymic, cervical, bone/joint, pancreatic, prostate, testicular, and epipharyngeal cancers.6,8,10,12,14,18,24
Because DM is associated with a variety of malignancies, screening DM patients is challenging. Consensus guidelines have not developed around malignancy screening in DM patients. Age-appropriate guidelines as outlined by the United States Preventive Services Task Force (USPSTF) and the American Cancer Society (ACS) (Table 1), recommended for DM patients, may not evaluate organs susceptible to malignancy in DM.26,27 In addition, a recently published retrospective analysis of a large cohort of DM patients in the US found that most cancers diagnosed after the onset of DM were identified using blind screening modalities, rather than guidance by symptoms or age appropriate screenings, further emphasizing the need for the development of screening guidelines in this population.28
This study was conducted to determine the incidence and types of malignancies in DM patients and the percentage of cancers not detected by routine age- and gender-appropriate malignancy screening in one of the largest samples of DM patients studied in the United States. We hope this research further supports the need for more rigorous screening methods in DM patients.
Table 1. Guidelines for performing age-appropriate malignancy screening as outlined by the USPSTF and the ACS
|
Type |
USPSTF |
ACS |
|
Breast |
50 to 75 years old: Biennial screening in women. |
Offer to women at 40 years old and recommend yearly screening at 45. |
|
Colorectal |
50 to 75 years old: Every 10 years. |
45 to 75 years old: Every 10 years. |
|
Cervical |
21 to 39 years old: Every 3 years with cervical cytology alone. 30 to 65 years: Screening every 3 years with cervical cytology alone, every 5 years with high-risk human papillomavirus (hrHPV) testing alone, or every 5 years with hrHPV testing in combination with cytology (cotesting). |
21 to 29 years old: Every 3 years with cervical cytology alone. 30 to 65 years old: Cervical cytology plus an HPV test every 5 years. |
|
Lung |
55 to 80 years old: Yearly low-dose CT (LDCT) chest for current smokers with 30 pack/year smoking history or those who have quit in the last 15 years. |
55 to 74 years old: Yearly low-dose CT (LDCT) chest for current smokers with a 30 pack-year history or those who have quit in the past 15 years. |
Materials and Methods
Data Source
Using the MarketScan® (MS) Commercial Claims and Encounters database, a retrospective cohort study from January 1, 2005 to December 31, 2015 was conducted. The database includes healthcare claims from more than 130 payers reporting healthcare utilization and expenditures, with claims from inpatient encounters, outpatient encounters and prescribed drugs, for an estimate of 150 million employees and family members per year. The database includes diagnostic codes (ICD-9), procedure codes (Current Procedural Terminology, 4th Edition (CPT-4)), dates of service, type of plan and expenditure data from 2005-2015. Patients’ ages ranged from birth to 64 years old. Individuals with Medicare or Medicaid are not included in the database. IRB approval was not necessary as all data is de-identified and therefore does not meet the definition of a human subject.
Study Design and Population
The DM cohort was formed from the MS database by identifying any patient receiving at least two separate diagnoses of DM by ICD-9 code (710.3), separated by at least six months. This technique has been previously validated by Kwa et al.29 The Index Date of diagnosis was defined as the first entry of ICD-9 code 710.3 for an individual patient. Patients were included if they had at least two years of continuous enrollment before and three years after the Index Date for DM diagnosis in order to capture the period of increased malignancy risk as noted by András et el.24 Any patient with any entry of ICD-9 codes for polymyositis or lupus were excluded. The control cohort was formed from the MS database by identifying patients without a history of DM. Controls were age and gender matched in a 5:1 control case ratio in order to increase the power of the study. The ICD-9 codes listed in Table 2 were used to identify specific cancers in DM and control patients. Patients with cancer prior to DM diagnosis were excluded.
Analyses
Controls were age and gender matched 5:1 with all DM cases in order to increase the power of the study. We compared the frequency and types of malignancies in patients <50 years old and those >50. Malignancies were separated by those screened for vs those not routinely screened for based on USPSTF/ACS guidelines in Table 1. The frequency of malignancy between DM cases and controls were compared using a chi-square test with odds ratios.
Table 2. ICD-9 Codes
|
Study |
ICD-9 code(s) |
Study |
ICD-9 code(s) |
|
Dermatomyositis |
710.3 |
Malignant neoplasm of connective and other soft tissue |
171 |
|
Malignant neoplasm of the brain |
191 |
Malignant melanoma of skin |
172 |
|
Malignant neoplasm of the trachea, bronchus, and lung |
162 |
Lymphosarcoma and reticulosarcoma and other specified malignant tumors of lymphatic tissue |
200 |
|
Malignant neoplasm of pleura |
163 |
Hodgkin's disease |
201 |
|
Malignant neoplasm of esophagus |
150 |
Other malignant neoplasms of lymphoid and histiocytic tissue |
202 |
|
Malignant neoplasm of stomach |
151 |
Multiple myeloma and immunoproliferative neoplasms |
203 |
|
Malignant neoplasm of small intestine, including duodenum |
152 |
Lymphoid leukemia |
204 |
|
Malignant neoplasm of colon |
153 |
Myeloid leukemia |
205 |
|
Malignant neoplasm of liver and intrahepatic bile ducts |
155 |
Monocytic leukemia |
206 |
|
Malignant neoplasm of gallbladder and extrahepatic bile ducts |
156 |
Other specified leukemia |
207 |
|
Malignant neoplasm of female breast |
174 |
Leukemia of unspecified cell type |
208 |
|
Malignant neoplasm of male breast |
175 |
Malignant neoplasm of other endocrine glands and related structures |
194 |
|
Secondary malignant neoplasm of other specified sites |
198 |
Malignant neoplasm of eyeball, except conjunctiva, cornea, retina, and choroid |
190 |
|
Malignant neoplasm of ovary and other uterine adnexa |
183 |
Malignant neoplasm cranial nerve |
192 |
|
Malignant neoplasm of prostate |
185 |
Malignant neoplasm of kidney, except pelvis |
189 |
|
Malignant neoplasm of testis |
186 |
Malignant neoplasm of uterus, part unspecified |
179 |
|
Malignant neoplasm of bone and articular cartilage |
170 |
Neuroendocrine tumors |
209 |
Results
632 total DM cases were identified across all age groups. 302 DM patients were >50 years old. 89 malignancies were identified in 44 DM patients. In 1526 controls, 222 malignancies were identified in 116 individuals. Malignancy frequency was 14.6% in DM patients compared to 7.6% in controls (p< 0.00001). 55% of malignancies in DM patients and 37.4% of malignancies in controls occurred in areas not routinely screened by ACS/USPSTF. When compared to controls, there was a statistically significant increase in DM malignancies not routinely screened (p < 0.00001). This included lymphomas, leukemias, prostate, skin, secondary, connective and soft tissue, ovarian, stomach, brain, reticulosarcoma, pancreas, liver, and neuroendocrine malignancies. (Figure 1)
330 DM patients were under 50 years old. 28 malignancies were identified in 21 DM patients. In 1,634 controls, 84 malignancies were identified in 46 individuals. Malignancy frequency was 6.4% in DM patients compared to 2.8% in controls (p=0.0010). 100% of malignancies in DM patients and 48% of malignancies in controls occurred in areas not routinely screened by ACS/USPSTF. When compared to controls, there was a statistically significant increase in DM malignancies not routinely screened (p < 0.00001). This included lymphoma, breast, ovary, kidney, secondary, endocrine, connective and soft tissue, reticulosarcoma, lymphosarcoma, brain, pancreas, stomach, prostate malignancies. (Figure 2)
Discussion
Lack of evidence-based guidelines for malignancy screening in DM represents a practice gap in both dermatology and rheumatology. Previous studies have confirmed an increased risk of malignancy in the DM population but currently there are no specific screening guidelines for DM patients. Here, we sought to determine if routine age- and gender-appropriate screening, as recommended by the USPSTF and ACS, would accurately evaluate the organs susceptible to malignancy in DM. Our results confirm that DM patients are at an increased risk of malignancy and highlight the unique risk faced by DM patients, particularly in organs not routinely evaluated. This risk is especially highlighted in patients traditionally deemed lower risk for cancer (<50 years old).
Increased age is consistently cited as the leading risk factor for developing malignancy, with risk rising more rapidly in midlife.30 White, et al. notes that the cumulative risk for all cancers combined increases with age, up to age 70, then decreases slightly.31 In a study looking at the future of cancer incidence in the United States, researchers found that from 2010 – 2030 cancer incidence will increase by 43% with a 63% increase in older adults and an 11% increase in younger adults.32 These findings have become the basis for screening guidelines, such as those provided by the USPSTF and ACS, leading to an emphasis on screening the adult population over 50 years old. However, for DM patients, these guidelines may be inadequate.
Currently, no formal consensus exists as to the best practices for malignancy screening in DM and some argue that only age-appropriate malignancy screening is necessary, especially with increasing years since initial diagnosis.33 Our results, however, show that age- appropriate screening misses a significant amount of malignancies in DM patients of all ages and, in patients under 50 years old, all malignancies were missed. This suggests that utilizing USPSTF and ACS guidelines for the DM population is highly likely to miss most, if not all, malignancies in DM patients, especially those under 50 years old.
Additionally, the USPSTF and ACS only recommend screening for breast, colorectal, cervical and lung cancers as there is an increased risk of these types of cancers in the general population and mechanisms exist to screen for these cancers.26,27 Our results, however, show that the types of malignancies seen in the DM population differ vastly from the general population. In DM patients of all ages, we found an increase in lymphomas and leukemias, prostate, lung, ovarian, pancreas, liver, and neuroendocrine tumors. In patients under 50 we found an increase in lymphomas, secondary, endocrine, leukemias, pancreas, stomach, and prostate cancers. Screening tests including the prostate specific antigen (PSA) for prostate cancer, CA 19-9 and carcinoembryonic antigen (CEA) blood tests for pancreatic cancer, transvaginal ultrasound and CA-125 blood test for ovarian cancer, endoscopy and serum pepsinogen levels for stomach cancer, alpha-fetoprotein (AFP) blood tests and ultrasound for liver cancer exist but are not used in the general population as the benefits do not outweigh the risks and their sensitivities/specificities are low.34-39 Utilization of these screening mechanisms in the DM population has not been well studied.
Researchers have looked into optimal screening mechanisms for patients with DM. In one study, Selva-O’Callaghan et al. assessed the utility of a PET scan for diagnosing occult malignancy in patients with myositis. After comparing a broad panel of gender-specific screening tests to a single PET-CT they found that the efficacy of the two screening options were comparable.40 Other studies suggest that the co-occurrence of other risk factors such as dysphagia, skin necrosis, cutaneous vasculitis, rapid onset of DM (< 4 weeks), elevated CK, an increase in ESR and a high level of C-reactive protein increase the risk of cancer development in patients with DM.41 In a study conducted by Leatham et al., they found that malignancy in DM patients is often not associated with a specific sign or symptom and that blind screening may be more effective in diagnosing malignancy.28
Myositis specific antibodies (MSA’s) can also provide insight into malignancy risk and may be utilized as a potential screening mechanism. Research has shown that patients with DM positive for anti-p155, anti-transcriptional intermediary factor 1γ (TIF-gamma) or anti-nuclear matrix protein 2 (NXP-2) antibodies have an increased risk of malignancy.42,43 In the absence of official screening recommendations, Selva-O’Callaghan et al. suggests baseline broad screening in all DM patients, with yearly repeat screenings in TIF-1gamma and NXP-2 positive patients, as well as those without detectable MSA’s, for 3-5 years.44 To date, there are no official recommendations as to best practices screening, but our results emphasize that consideration should be given to young patients as guidelines undergo development.
Limitations
Our study has several limitations. First, it does not distinguish blind versus targeted diagnosis of cancer. This information would better help us understand the modalities in which the malignancies were diagnosed and help to guide screening in future patients. Second, even though the sample size was large, the MarketScan® database only includes patients up to 64 years old. Additionally, no Medicare or Medicaid patients were included, therefore the actual population risk of cancer is likely underestimated, especially in those >50, and the generalizability of our study is limited. Lastly, we are unable to account for the presence or absence of multiple known risk factors, including clinical signs, MSAs, or inflammatory markers which are associated with increased risk of malignancy in DM patients.
Conclusion
Our results agree with the well-defined risk for neoplasia with DM and highlight that younger patients have a considerably higher risk of developing cancer with DM than matched controls, especially in organs not routinely screened. Development of evidence-based guidelines to optimize malignancy screening in adult DM patients of all ages is needed. We agree with previously published recommendations for baseline broad screening in all DM patients, with yearly repeat screenings in TIF-1gamma and NXP-2 positive patients, as well as those without detectable MSA’s, for 3-5 years.44 Broad malignancy screening should include modalities for identifying cancers poorly visualized on traditional imaging, such as ovarian and prostate. Such screening should not be relegated to older patients and special attention should be given to young DM patients as guidelines undergo development. Overall, our research supports the need for future studies aimed at optimizing malignancy screening in all patients with DM.
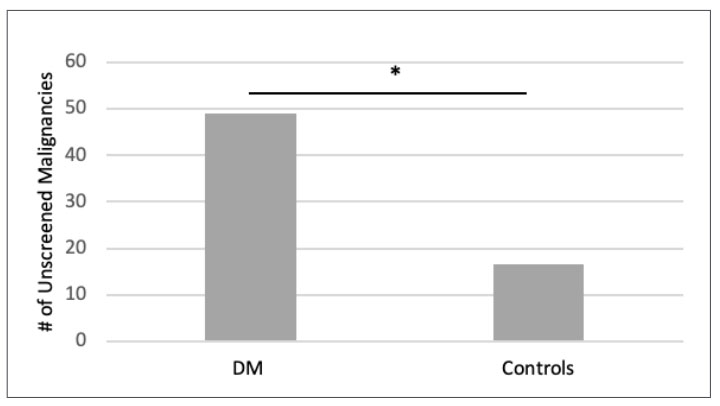
Figure 1. Number of unscreened malignancies in DM patients and controls of >50 [controls scaled by 1/5]. Number of malignancies not routinely screened by ACS/USPSTF guidelines in DM patients and controls of >50 years old. There was a statistically significant (*) increase in the number of unscreened malignancies in DM patients when compared to age-and-gender matched controls. 55% of malignancies in DM patients occurred in organs not routinely screened. This included lymphomas, leukemias, prostate, skin, secondary, connective and soft tissue, ovarian, stomach, brain, reticulosarcoma, pancreas, liver, and neuroendocrine malignancies. |
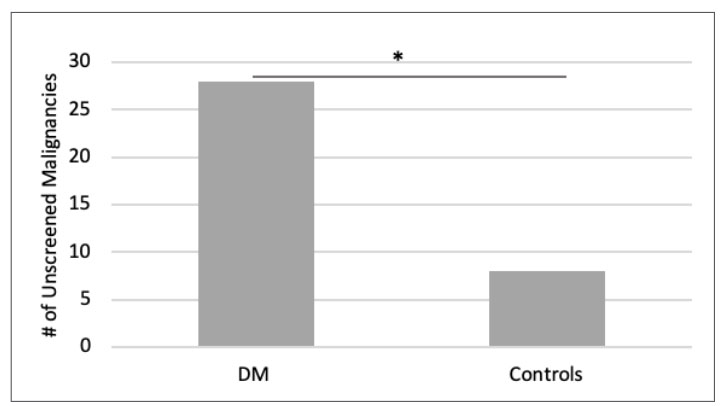
Figure 2. Number of unscreened malignancies in DM patients and controls of <50 [controls scaled by 1/5]. Number of malignancies not routinely screened by ACS/USPSTF guidelines in DM patients and controls of <50 years old. There was a statistically significant (*) increase in the number of unscreened malignancies in DM patients when compared to age-and-gender matched controls. 100% of malignancies in DM patients occurred in organs not routinely screened. This included lymphoma, breast, ovary, kidney, secondary, endocrine, connective and soft tissue, reticulosarcoma, lymphosarcoma, brain, pancreas, stomach, prostate malignancies. |
Author Information
Corresponding Author
*Sarah Kitts, BS
skitts@pennstatehealth.psu.edu
Author Contributions
All author(s) have given approval to the final version of the manuscript.
Funding Sources
This research was funded by the James and Joyce Marks Educational Endowment.
Disclosures
Nancy Olsen, MD (Malinckrodt Pharmaceuticals)
Acknowledgements
Data was originally published as a research letter in the British Journal of Dermatology. Appropriate permissions were obtained to publish in The Penn State Journal of Medicine.
References
1. Krathen, M. S., Fiorentino, D., & Werth, V. P. (2008). Dermatomyositis. Current directions in autoimmunity, 10, 313–332. https://doi.org/10.1159/000131751
2. Furst DE, Amato AA, Iorga SR, Gajria K, Fernandes AW. Epidemiology of adult idiopathic inflammatory myopathies in a U.S. managed care plan. Muscle Nerve. 2012;45(5):676-83.
3. G. S. Polymyositis. Berliner Klinische Wochenshrift. 1916;53:489.
4. H. K. Uber Primaire nichteitrige Polymyositis. Dtsch Arch Klin Med. 1916;120:335-9.
5. Yang Z, Lin F, Qin B, Liang Y, Zhong R. Polymyositis/dermatomyositis and Malignancy Risk: A Metaanalysis Study. The Journal of Rheumatology. 2015;42(2).
6. Chen Y-J, Wu C-Y, Huang Y-L, Wang C-B, Shen J-L, Chang Y-T. Cancer risks of dermatomyositis and polymyositis: a nationwide cohort study in Taiwan. Arthritis Research & Therapy. 2010;12(2).
7. Buchbinder R, Forbes A, Hall S, Dennett X, Giles G. Incidence of Malignant Disease in Biopsy-Proven Inflammatory Myopathy. Annals of Internal Medicine. 2001;134(12):1087-95.
8. Hill C, Zhang Y, Sigurgeirsson B, Pukkala E, Mellemkjaer L, Airio A, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357:96-100.
9. Kuo C, See L, Yu K, Chou I, Chang H, Chiou M, et al. Incidence, cancer risk and mortality of dermatomyositis and polymyositis in Taiwan: a nationwide population study. British Journal of Dermatology. 2011;165:1273-9.
10. Mok CC, To CH, Ying KY. Standardized Incidence Ratios and Predictors of Malignancies in 215 Southern Chinese Patients with Inflammatory Myopathies. Arthritis & Rheumatism. 2012;64.
11. Antiochos B, Brown L, Li Z, Tosteson T, Wortmann R, Rigby W. Malignancy Is Associated with Dermatomyositis But Not Polymyositis in Northern New England, USA. Journal of Rheumatology. 2009;36(12).
12. Azuma K, Yamada H, Ohkubo M, Yamasaki Y, Yamasaki M, Mizushima M, et al. Incidence and predictive factors for malignancies in 136 Japanese patients with dermatomyositis, polymyositis and clinically amyopathic dermatomyositis. Modern Rheumatology. 2010;21:178-83.
13. Liu WC, Ho M, Koh W-P, Tan A, Ng P, Chua S, et al. An 11-year Review of Dermatomyositis in Asian Patients. Annals of the Academy of Medicine, Singapore. 2010;39:843-7.
14. Stockton D, Doherty V, Brewster D. Risk of cancer in patients with dermatomyositis or polymyositis, and follow-up implications: a Scottish population-based cohort study. British Journal of Cancer. 2001;85(1):41-5.
15. Fang Y-F, Jan Wu Y-J, Kuo C-F, Luo S-F, Yu K-H. Malignancy in dermatomyositis and polymyositis: analysis of 192 patients. Clinical Rheumatology. 2016;35:1977-84.
16. Titulaer M, Soffietti R, Dalmau J, Gilhus N, Giometto B, Graus F, et al. Screening for tumours in paraneoplastic syndromes: report of an EFNS Task Force. European Journal of Neurology. 2011;18(1).
17. Wang J, Guo G, Chen G, Wu B, Lu L, Bao L. Meta-analysis of the association of dermatomyositis and polymyositis with cancer. British Journal of Dermatology. 2013;169:838-47.
18. Limaye V, Luke C, Tucker G, Hill C, Lester S, Blumbergs P. The incidence and associations of malignancy in a large cohort of patients with biopsy-determined idiopathic inflammatory myositis. Rheumatology International. 2012;33:965-71.
19. Prohic A, Kasumagic-Halliovic E, Simic D, Selmanagic A. Clinical and biological factors predictive of malignancy in dermatomyositis. Journal European Academy of Dermatology and Venereology. 2009;23:591-2.
20. Sparsa A, Liozon E, Herrmann F, Ly K, Lebrun V, Soria P, et al. Routine vs Extensive Malignancy Search for Adult Dermatomyositis and Polymyositis. JAMA Dermatology. 2002;138.
21. Zhang W, Jiang SP, Huang L. Dermatomyositis and malignancy: a retrospective study of 115 cases. Eur Rev Med Pharmacol Sci. 2009;13(2):77-80.
22. Fardet L, Dupuy A, Gain M, Kettaneh A, Cherin P, Bachelez H, et al. Factors associated with underlying malignancy in a retrospective cohort of 121 patients with dermatomyositis. Medicine (Baltimore). 2009;88(2):91-7.
23. Madan V, Chinoy H, Griffiths CEM, Cooper RG. Defining cancer risk in dermatomyositis. Part 1. Clinical Dermatology. 2009 Jun;34(4):451-5.
24. Andras C, Ponyi A, Constantin T, Csiki Z, Eva S, Szodoray P, et al. Dermatomyositis and polymyositis associated with malignancy: a 21-year retrospective study. The Journal of Rheumatology. 2008;35(3):438-44.
25. Sigurgeirsson B, Lindelof B, Edhag O, Allander E. Risk of cancer in patients with dermatomyositis or polymyositis. A population-based study. N Engl J Med. 1992;326(6):363-7.
26. https://www.uspreventiveservicestaskforce.org/Page/Name/uspstf-a-and-b-recommendations/
28. Leatham H, Schadt C, Chisolm S, Fretwell D, Chung L, Callen JP, et al. Evidence supports blind screening for internal malignancy in dermatomyositis: Data from 2 large US dermatology cohorts. Medicine (Baltimore). 2018;97(2):e9639.
29. Kwa MC, Ardalan K, Laumann AE, Nardone B, West DP, Silverberg JI. Validation of international classification of diseases codes for the epidemiologic study of dermatomyositis. Arth Care Res. 2017;69(5):753-7
30. U.S. Cancer Statistics Working Group. US cancer statistics: 1999–2009 incidence and mortality web-based report. Atlanta GA: USDHHS, CDC; 2013. www.cdc.gov/uscs.
31. White MC, Holman DM, Boehm JE, Peipins LA, Grossman M, Henley SJ. Age and cancer risk: a potentially modifiable relationship. Am J Prev Med. 2014;46(3 Suppl 1):S7-15.
32. Smith BD, Smith GL, Hurria A, Hortobagyi GN, and Buchholz TA. uture of Cancer Incidence in the United States: Burdens Upon an Aging, Changing Nation. Journal of Clinical Oncology 2009 27:17, 2758-2765
33. Chow, W., Gridley, G., Mellemkjær, L., Mclaughlin, J.K., Olsen, J.H., & Fraumeni, J.F. (1995). Cancer risk following polymyositis and dermatomyositis: a nationwide cohort study in Denmark. Cancer Causes & Control, 6, 9-13.
34. https://www.mayoclinic.org/tests-procedures/psa-test/in-depth/prostate-cancer/art-20048087
35. https://www.cancer.org/cancer/pancreatic-cancer/detection-diagnosis-staging/detection.html
36. https://www.cancer.org/cancer/ovarian-cancer/detection-diagnosis-staging/detection.html
37. https://www.cancer.gov/types/stomach/patient/stomach-screening-pdq
38. https://www.cancer.org/cancer/liver-cancer/detection-diagnosis-staging/detection.html
39. https://www.cancer.gov/about-cancer/screening
40. Selva-O’Callaghan A, Grau J, Gamez-Cenzano C, Vidaler-Palacin A, Martinex-Gomez X, Trallero-Araguas E, et al. Conventional Cancer Screening versus PET/CT in Dermatomyositis/Polymyositis. The American Journal of Medicine. 2010;123:558-62.
41. Jakubaszek M, Kwiatkowska B, Maślińska M. Polymyositis and dermatomyositis as a risk of developing cancer. Reumatologia. 2015;53(2):101-5.
42. Trallero-Araguás E, Rodrigo-Pendás JÁ, Selva-O’Callaghan A, Martínez-Gómez X,Bosch X, Labrador-Horrill M, et al. Usefulness of anti-p155 autoantibody for diagnosing cancer-associated dermatomyositis: a systematic review and meta-analysis. Arthritis Rheum 2012; 64: 523–32.
43. Fiorentino DF, Chung LS, Christopher-Stine L, Zaba L, Li S, Mammen AL,et al. Most patients with cancer-associated dermatomyositis have antibodies to nuclear matrix protein NXP-2 or transcription intermediary factor 1γ. Arthritis Rheum 2013; 65: 2954–62.
44. Selva-O’Callaghan A, Martinez-Gómez X, Trallero-Araguás E, Pinal-Fernández I. The diagnostic work-up of cancer-associated myositis. Current Opinion Rheumatology. 2018 Nov;30(6):630-636.