Figure 1. Ulcer with violaceous rolled borders, purulent base and granulation tissue before treatment with ustekinumab (2/17/2020).
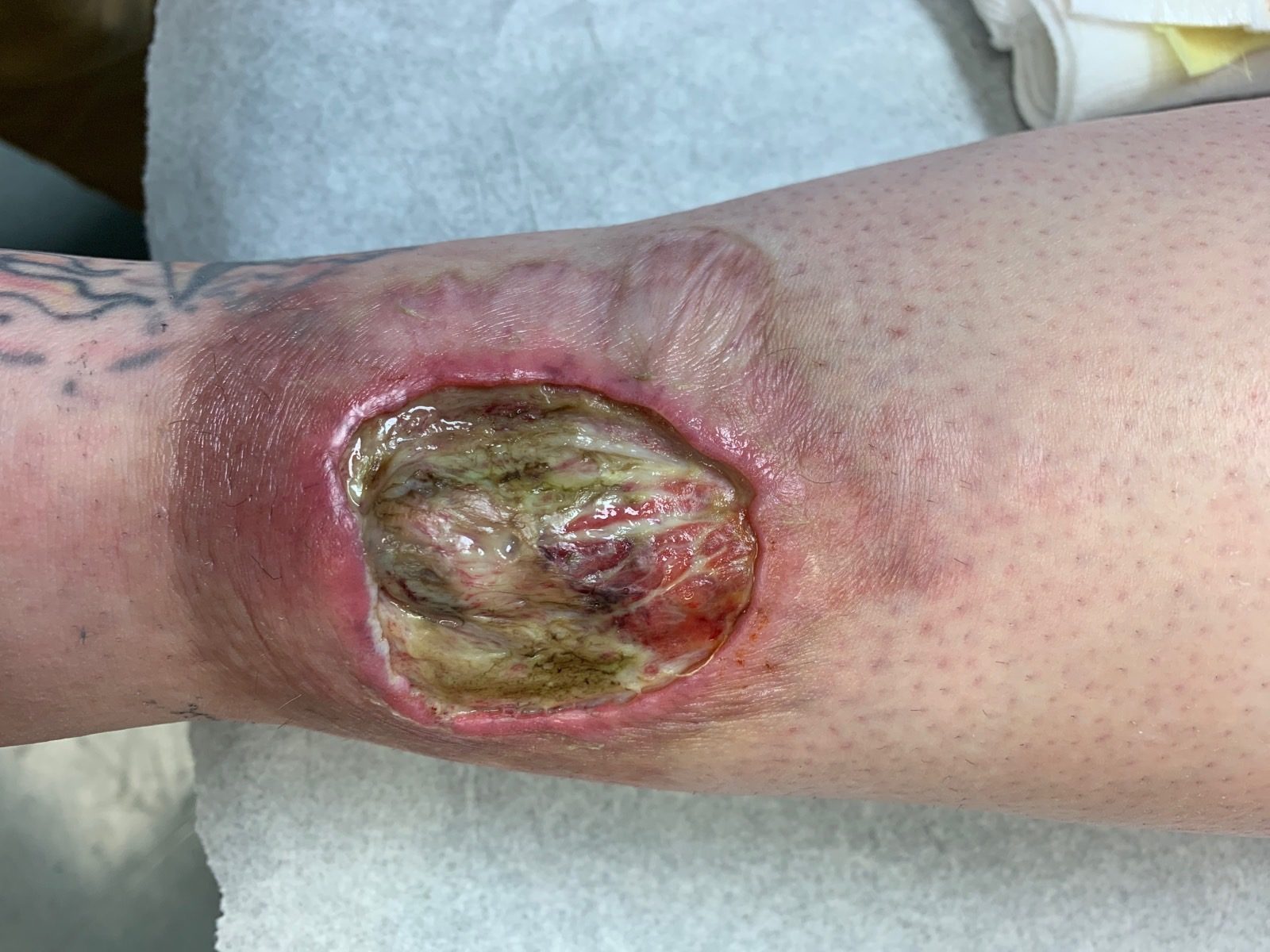
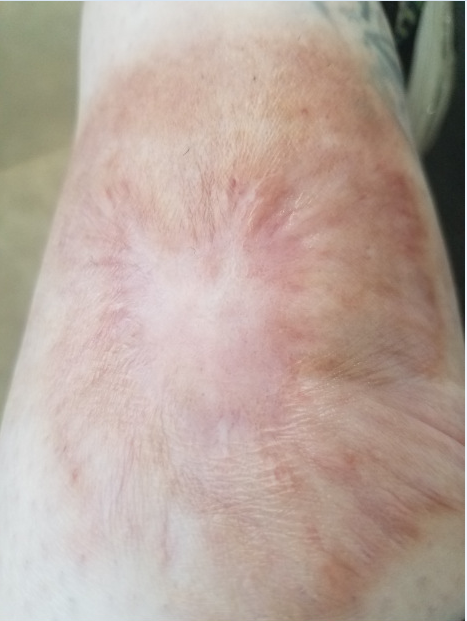
Figure 2. Healed ulcer with scarring after more than a year of treatment with ustekinumab (6/28/2021).
*Nanjiba Nawaz1, Marisa Riley1, Matthew Helm2
1Pennylvania State College of Medicine, Hershey, PA, USA; 2Penn State Hershey Department of Dermatology, Hershey, PA, USA
KEYWORDS: Neutrophilic Dermatosis, Pyoderma Gangrenosum, Ustekinumab, Biologic
Learning Objectives
1. Present a patient with pyoderma gangrenosum (PG) refractory to multiple therapies.
2. Discuss PG development, treatment approaches, and challenges.
3. Highlight ustekinumab as a possible treatment for refractory PG.
Case Description
A 38-year-old female with a history of diabetes, deep venous thrombosis, and non-alcoholic fatty liver disease presented to the Dermatology clinic for PG refractory to treatment. She first presented in 2015 for ulcer formation with light purple rolled borders and purulent base, granulation tissue and visible tendons. Treatment was initiated with oral mycophenolate mofetil and cyclosporine, but due to inadequate response transitioned to infliximab, minocycline, and prednisone taper. Her condition improved on this regimen and within 2 years her wound healed completely. The infliximab infusions were then discontinued.
A year later, four new wounds appeared on her legs, and she restarted infliximab, minocycline, and prednisone taper. An extensive work up for underlying causes such as autoimmune disease, inflammatory bowel disease (IBD), medication changes and malignancy came back unremarkable, deeming her PG idiopathic. She continued to improve, with full wound closure within a year.
In December, 2019 her ulcer reopened (Figure 1), and she subsequently started cyclosporine and prednisone then infliximab, with minimal response. She also developed resistant infection of the wound with Pseudomonas, which required treatment with intravenous antibiotics. In March of 2020, she then began treatment with ustekinumab at 90 mg/kg every 12 weeks, which was subsequently changed to every 6 weeks in September of 2020 after the patient experienced a decreased response on the former regimen. Her ulcer is slowly healing with this regimen, with significant reduction in span and depth as of June 2021 (Figure 2).
Figure 1. Ulcer with violaceous rolled borders, purulent base and granulation tissue before treatment with ustekinumab (2/17/2020).

Figure 2. Healed ulcer with scarring after more than a year of treatment with ustekinumab (6/28/2021).

Discussion
PG is a non-infectious neutrophilic dermatosis with a painful, ulcerating course1. The exact pathophysiology remains unknown, but may involve loss of innate immune regulation and altered neutrophil chemotaxis2. PG is often associated with underlying systemic conditions such as IBD, autoimmune disease, medication reaction, and lymphoproliferative disorders. About one half of the time its cause remains idiopathic, such as with our patient3. Diagnosis is primarily clinical and may be supported by biopsy. Early diagnosis and effective lesion treatment help prevent further complications, including secondary bacterial infection and sepsis4.
Currently, no gold standard treatment exists for PG, and combination therapy along with good wound care is an accepted strategy to address the development and progression of ulcers5. Traditionally, antineutrophilic agents such as dapsone and colchicine and immunosuppressing agents such as systemic steroids, mycophenolate mofetil, cyclosporine and anti-TNF inhibitors are tried first6. Recent evidence showed elevated expression of interleukin(IL)‐23 in patients’ skin with PG compared with healthy skin, lending support for possible treatment with ustekinumab, a human monoclonal antibody that targets IL‐12 and IL‐23 cytokines, specifically the p40 subunit. Subsequently, ustekinumab may hinder the activity of Th1 and Th17 cells which interferes with the body’s inflammatory response7. There have been several cases of PG successfully treated with ustekinumab8,9.
Here we reported a patient with a refractory ulcerating PG without associated underlying systemic illness, and a disease course refractory to multiple other drug trials for whom the IL-12/23 monoclonal antibody ustekinumab was both successful and well-tolerated. PG presents a challenge for many providers in the heavy toll disease course has on patients, and the low levels of evidence for current proposed treatment options. This case suggests ustekinumab as an additional biological therapy in cases of refractory PG.
Author Information
Corresponding Author
* Nanjiba Nawaz, BS
nnawaz@pennstatehealth.psu.edu
Author Contributions
All author(s) have given approval to the final version of the manuscript.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Disclosures
No authors have any disclosures or conflicts of interest at this time.
Acknowledgements
None.
References
1.Wollina, U. (2007). Pyoderma gangrenosum–a review. Orphanet journal of rare diseases, 2(1), 1-8.
2.Braswell, S. F., Kostopoulos, T. C., & Ortega-Loayza, A. G. (2015). Pathophysiology of pyoderma gangrenosum (PG): an updated review. Journal of the American Academy of Dermatology, 73(4), 691-698.
3.Ahronowitz, I., Harp, J., & Shinkai, K. (2012). Etiology and management of pyoderma gangrenosum. American journal of clinical dermatology, 13(3), 191-211.
4.Bennett, M. L., Jackson, J. M., Jorizzo, J. L., Fleischer Jr, A. B., White, W. L., & Callen, J. P. (2000). Pyoderma gangrenosum. A comparison of typical and atypical forms with an emphasis on time to remission. Case review of 86 patients from 2 institutions. Medicine, 79(1), 37-46.
5.Patel, F., Fitzmaurice, S., Duong, C., He, Y., Fergus, J., Raychaudhuri, S. P., Shirakawa Garcia, M., & Maverakis, E. (2015). Effective strategies for the management of pyoderma gangrenosum: a comprehensive review. Acta dermato-venereologica, 95(5), 525-533.
6. Quist, S. R., & Kraas, L. (2017). Treatment options for pyoderma gangrenosum. JDDG: Journal der Deutschen Dermatologischen Gesellschaft, 15(1), 34-40.
7. Guenova, E., Teske, A., Fehrenbacher, B., Hoerber, S., Adamczyk, A., Schaller, M., ... & Biedermann, T. (2011). Interleukin 23 expression in pyoderma gangrenosum and targeted therapy with ustekinumab. Archives of dermatology, 147(10), 1203-1205.
8.Vallerand, I. A., & Hardin, J. (2019). Ustekinumab for the treatment of recalcitrant pyoderma gangrenosum: a case report. SAGE open medical case reports, 7, 2050313X19845206.
9.Low, Z. M., & Mar, A. (2018). Treatment of severe recalcitrant pyoderma gangrenosum with ustekinumab. Australasian Journal of Dermatology, 59(2), 131-134.