Treatment of Calcinosis Cutis Secondary to Juvenile Dermatomyositis with Intralesional Sodium Thiosulfate Injections
Stephanie Golub, MS1, Taylor E. Gladys1, Matthew Helm, MD2
1Penn State College of Medicine, Hershey, PA; 2Department of Dermatology, Penn State College of Medicine, Hershey, PA
KEYWORDS: dermatomyositis, STS, calcinosis cutis
Learning Objectives
1. Calcinosis cutis (CC) as a complication of juvenile dermatomyositis (JDM)
2. Sequalae of CC
3. Sodium thiosulfate (STS) injections for treatment of severe, refractory CC
Case Description
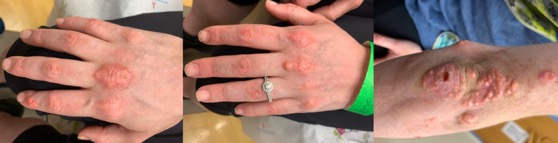
A 23-year-old female with a 16-year history of JDM categorized by elevated creatinine kinase (2400units/L), lactate dehydrogenase (404units/L), AST (145units/L), ALT (102units/L) and aldolase (25units/L); myositis, dysphagia, lipodystrophy, Gottron’s papules and dystrophic calcinosis cutis (CC), was referred for management of subcutaneous calcification of the hands and elbow (Figure 1A). Her JDM was well controlled on abatacept (750mg IV qmonth), colchicine (0.6mg PO tid), hydroxychloroquine (200mg PO bid), immune globulin intravenous (IVIG) (2g/kg per month) and methylprednisolone (500mg IV qmonth). Calcinosis was the most troubling manifestation of her JDM at the time which developed 10 years post diagnosis and progressed rapidly to involve much of her body, especially joints. Pain from these nodules proved difficult to manage.
Over a 6-year period, treatment with methotrexate, rituximab, IV pamidronate, diltiazem, mycophenolate mofetil, tacrolimus, topical creams and intralesional steroid injections failed. As her condition worsened, she developed depression with suicidal ideation, and her quality of life diminished.
Discussion
CC is the deposition of insoluble calcium crystals in the skin and subcutis1,2. It is often precipitated by underlying autoimmune connective tissue diseases (ACTDs) like JDM, where CC can be severe3.
Sequelae of CC include pain, ulceration, functionality loss and infection.1,2,3 While aggressive management of ACTDs has improved symptom development, treatment remains challenging as no current guidelines exist1,2,3. Options include calcium channel blockers, colchicine, IVIG, bisphosphonates, methotrexate, rituximab, cyclophosphamide, surgical interventions and STS1,3.
STS is an inorganic salt with antioxidant, reducing and chelating properties used to treat cyanide poisoning and calcium-related disorders4-7. It has many proposed mechanisms including formation of soluble calcium thiosulfate and stimulation of vasodilation via antioxidant and reducing properties, restoring endothelial function4,5,7. Although originally delivered topically and intravenously, diffuse distribution of lesions and poor side effect profiles initiated intralesional injections1-4. This improves accessibility of the target, lowers complication risk and systemic effects.4 Limitations include inability to treat lesions deep to soft tissue and local pain or infection4,6. There are no contraindications for STS injections, but side effects include hypotension, headache and disorientation8.
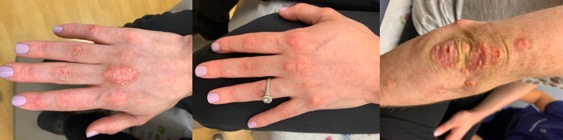
Five mL of 250 mg/mL of STS mixed in normal saline were injected into lesions on the right and left middle metacarpophalangeal joints and right index finger distal interphalangeal joint. Two weeks later, the treated areas softened and decreased in size. The same protocol was repeated with three additional injections to the elbow. In total, four rounds of STS were injected into the hands and three into the elbow in two-week intervals. Nodules improved with decreases in size, pain and rigidity, improving range of motion and joint mobility (Figure 1B). Our patient was happy with the results but did experience pain for 48 hours post-injection. While this treatment significantly improved CC symptoms, other JDM manifestations remained unchanged.
Mounting evidence describing successful response to intralesional STS in individual CC patients should be an impetus for controlled studies on the efficacy of this treatment. Through its multifactorial mechanism, STS reduces calcium deposition, alleviates pain and promotes healing. This quick outpatient treatment can provide significant relief and improve quality of life.
Author Information
Corresponding Author
Stephanie Golub, MS
sgolub@pennstatehealth.psu.edu
Author Contributions
All authors have given approval to the final version of the manuscript.
Funding Sources
All authors received no financial support for the research, authorship, and/or publication of this article.
Disclosures
No authors have any disclosures or conflicts of interest at this time.
Acknowledgements
None.
References
1. Traineau H., Aggarwal, R., Monfort, J. B., Senet, P., Oddis, C. V., Chizzolini, C., Barbaud, A., Francès, C., Arnaud, L., & Chasset, F. (2020). Treatment of calcinosis cutis in systemic sclerosis and dermatomyositis:
A review of the literature. Journal of the American Academy of Dermatology, 82(2), 317–325.
2. Gunasekera, N. S., Maniar, L., Lezcano, C., Laga, A. C., & Merola, J. F. (2017). Intralesional Sodium Thiosulfate Treatment for Calcinosis Cutis in the Setting of Lupus Panniculitis. JAMA dermatology, 153(9), 944–945.
3. Balin, S. J., Wetter, D. A., Andersen, L. K., & Davis, M. D. (2012). Calcinosis cutis occurring in association with autoimmune connective tissue disease: the Mayo Clinic experience with 78 patients, 1996-2009.
Archives of dermatology, 148(4), 455–462.
4. Strazzula, L., Nigwekar, S. U., Steele, D., Tsiaras, W., Sise, M., Bis, S., Smith, G. P., & Kroshinsky, D. (2013). Intralesional sodium thiosulfate for the treatment of calciphylaxis. JAMA dermatology, 149(8),
946–949.
5. Schlieper, G., Brandenburg, V., Ketteler, M., & Floege, J. (2009). Sodium thiosulfate in the treatment of calcific uremic arteriolopathy. Nature Reviews Nephrology, 5(9), 539-543.
6. Raffaella, C., Annapaola, C., Tullio, I., Angelo, R., Giuseppe, L., & Simone, C. (2009). Successful Treatment of Severe Iatrogenic Calcinosis Cutis with Intravenous Sodium Thiosulfate in a Child Affected by T-Acute
Lymphoblastic Leukemia. Pediatric Dermatology, 26(3), 311-315.
7. Vedvyas, C., Winterfield, L.S., & Vleugels, R.A. (2012). Calciphylaxis: A systematic review of existing and emerging therapies. Journal of the American Academy of Dermatology, 67(6), e253-260.
8. U.S. Food and Drug Administration/Center for Drug Evaluation and Research. (2012). Highlights of Prescribing Information: Sodium Thiosulfate Injections, USP.