Introduction
Obstructive sleep apnea (OSA) is a sleep disorder characterized by pauses in breathing of 10 seconds or more causing unrestful sleep and often accompanied by loud or abnormal snoring, daytime sleepiness, irritability, and depression. According to the National Sleep Foundation, more than 18 million Americans have OSA, 80% of whom are undiagnosed1. Undiagnosed OSA is associated with higher healthcare expenditures costing the United States an estimated 149.6 billion dollars in 2015 alone. Further, it accounts for the 4th highest percentage of healthcare expenses, trailing only cancer, diabetes and heart disease2. In addition to the financial costs, OSA increases morbidity and mortality of cardiovascular and cerebrovascular events3. Health systems and patients would benefit from timely diagnosis and treatment of OSA.
The STOPBANG survey was originally developed for use in perioperative patients to screen for OSA and stratify risk of complications from anesthesia.4 Since then, it has been utilized and validated in tertiary care centers to screen many diverse populations of patients for OSA5,6. However, much of the literature is limited to specific subpopulations such as obese patients7 and patients over 50,8 and thus may not be as generalizable. Further, there is limited published data identifying patient characteristics that may impact interest in pursuing follow-up polysomnography (PSG). This study aims to utilize the STOPBANG questionnaire to identify the prevalence of risk of undiagnosed OSA on an inpatient medicine ward, evaluate the interest of patients to undergo further testing and determine patient characteristics that may impact interest in pursuing follow-up PSG.
Methods
We conducted a single-center, descriptive study on adult patients at a tertiary care medical center by administering the STOPBANG survey to randomly selected patients on the Internal Medicine Service. The quick STOPBANG questionnaire is a validated tool for identifying high risk features of OSA.5 It is composed of 8 questions that ask about core symptoms and risk factors for OSA including snoring, excessive daytime sleepiness (EDS), choking during sleep, hypertension, body mass index (BMI), age, neck size and gender (Figure 1).
Figure 1. STOPBANG Questionnaire used during patient recruitment. Reprinted with permission from Dr. Chung and colleagues at STOPBANG.ca.
1. SNORING? Do you Snore Loudly (loud enough to be heard through closed doors or your bed-partner elbows you for snoring at night)? Yes/No
2. TIRED? Do you often feel Tired, Fatigued, or Sleepy during the daytime (such as falling asleep during driving or talking to someone)? Yes/No
3. OBSERVED? Has anyone Observed you Stop Breathing or Choking/Gasping during your sleep? Yes/No
4. PRESSURE? Do you have or are being treated for High Blood Pressure? Yes/No
5. BODY MASS INDEX more than 35 kg/m2?
6. AGE older than 50? Yes/No
7. NECK SIZE large? (Measured around Adam’s apple)
For male, is your shirt collar 17 inches / 43cm or larger?
For female, is your shirt collar 16 inches / 41cm or larger?
8. GENDER = Male? Yes/No
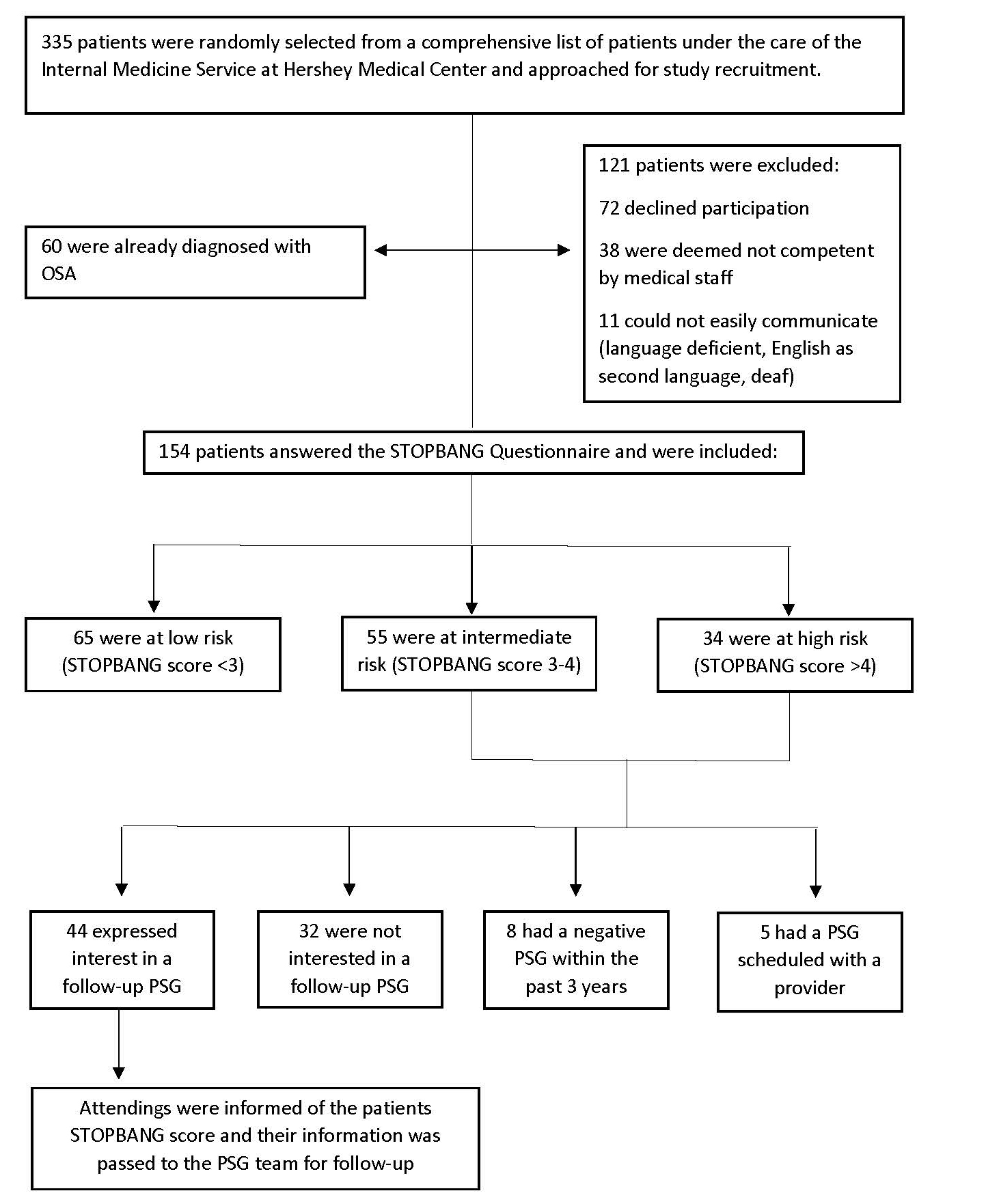
From August 6th to December 2nd 2019, an interviewer approached randomly selected patients on the Internal Medicine Service in their rooms and guided them through the consent form. Patients were excluded upon if they were under the age of 18, lacked capacity to consent, had significant language barriers, declined to participate or were currently diagnosed with OSA. After giving verbal consent, patients were administered the STOPBANG Questionnaire. If patients could not self-report neck size at the time of the interview, the interviewer measured it with paper measuring tape. If patients were uncertain of the answers to STOPBANG questions, such as treatment for or diagnosis of hypertension, the electronic medical record was utilized for verification. Each patient’s STOPBANG score was tabulated on site, and they were informed of their result and its implications for their risk of OSA. Patient’s with a score of 3-4 or >4 (indicating intermediate or high-risk) were recommended an outpatient PSG and asked if their contact information could be shared with the Sleep Medicine Program. The Human Subjects Protection Office determined this work did not require formal IRB review because the research met the criteria for exempt research according to institutional policies and federal regulations.
All variables were summarized prior to analysis by checking the distributions of continuous variables with histograms and normal probability plots. Statistical significance was set at p<0.05. Patients with a STOPBANG score of >2 and interest in undergoing PSG for definitive diagnosis were compared to subjects without interest in undergoing a PSG using a Chi-square test for categorical variables. For continuous variables, a two-sample t-test was used for normally distributed variables (age) and a Wilcoxon Rank Sum test was used for skewed variables (BMI). This analysis was extended to a multivariable logistic regression model that included the most significant factors of age, snoring, and neck size. Prior to fitting the multivariable model, the predictors were checked for multicollinearity using variance inflation factor (VIF) statistics. The fit of the model was assessed using Pearson, Deviance, and Hosmer & Lemeshow goodness-of-fit tests. Odds ratios were used to quantify the magnitude and direction of variables significantly associated with the willingness to pursue an OSA work-up in the multivariable model. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Three hundred thirty five patients were approached, while 121 patients were excluded because 38 were not competent to participate, 11 had language barriers and 72 declined to participate. 60 (17.9%) were already diagnosed with OSA and were excluded. Of the 154 patients interviewed, the average age was 60.7 and BMI was 28.7 kg/m2. Forty-one (26.6%) endorsed snoring, 44 (28.6%) endorsed EDS, 36 (23.4%) had been observed to stop breathing, 81 (52.6%) had diagnosed hypertension, 33 (21.4%) had a BMI over 35.0 kg/m2, 112 (72.7%) were over 50 years in age, 55 (36.2%) had a large neck size (>17 cm if male, >16 cm if female) and 72 (46.8%) of participants were male. 65 (42.2%), 55 (35.7%) and 34 (22.1%) participants were at low, intermediate and high risk of OSA, respectively (Table 1).
Of the 89 patients at intermediate to high risk, 44 (49.4%) expressed interest in following up with a PSG, 32 (36.0%) were not interested, 8 (9.0%) had a recent negative PSG, and 5 (5.6%) had recently scheduled a PSG (Figure 2). For patients that expressed interest, their attendings were informed of their STOPBANG score and their information was passed to the PSG team for follow-up.
In post-hoc analysis, we found that reported snoring on the STOPBANG questionnaire was significantly associated with interest in following up p=0.037. Younger patients were also more likely to express interest in following up p=0.004. All other answers to the STOPBANG questionnaire were not significantly associated with interest in pursuing PSG (Table 2), although those with a larger neck size tended toward a significant association with interest (p=0.084). The association between age and interest was confirmed through a two-sample t-test (p=0.001). The mean age of patients interested in undergoing PSG was 59.0 (95% CI 55.2-62.9) while the mean of patients not interested was 69.6 (95% CI 64.3-74.8) p=0.001.
To further explore these associations, we generated a logistical regression using age, endorsement of snoring and neck size. The model passed multicollinearity testing and showed good fit on Deviance, Pearson and Homer-Lemeshow goodness-of-fit testing. Age and snoring remained significant at p=0.031 and p=0.027, respectively, while neck size remained insignificant at p=0.170. Older patients had 10 times lower odds to be interested in follow-up (OR 0.098 95% CI: 0.012-0.893) and patients that endorsed snoring had 3 times higher odds to express interest in follow-up (OR 3.15 CI: 1.14-8.75).
Discussion
The primary finding of this study is a prevalence of intermediate to high risk of OSA (STOPBANG score >2) in 57.8% of a randomly sampled inpatient population on the hospitalist service at tertiary academic medical center. These results are lower than previous work by Kumar et al which found high risk on STOPBANG in 76.8% of patients6. This difference may be partially explained by differences in patient populations; Kumar’s study was conducted at an urban tertiary care center whereas our center serves a primarily suburban/rural catchment area. Finally, we found that 17.9% of our patients interviewed were already diagnosed with OSA, similar to the 15.9% that Kumar identified. Another study by Finkel et al. found the prevalence of high risk of OSA in undiagnosed patients undergoing surgery was 23.7% when screened with the apnea risk evaluation system9. The vast differences between our prevalence values and Finkel’s is likely due to differences in questionnaire stratification; the apnea risk evaluation system stratifies risk by no, low, moderate, and high. Thus, many patients fitting into moderate risk may have fit into the intermediate risk group on the STOPBANG survey. Furthermore, the patient population they surveyed was composed of peri-operative patients, whereas ours were on the internal medicine service.
Other work on inpatient populations has been limited to subpopulations. Sharma found that the prevalence of high OSA risk to be 84.4% and later 76.2% in patients with a BMI >30, which is lower than our rate of 90.9% in patients with a higher threshold for BMI of >357,10. Notably, Sharma opted to use the simplified STOP survey so that patients could self-administer the survey by omitting the additional four questions about BMI, age, neck size and gender. Shear et al found the prevalence of OSA in patients older than 50 to be 39.5% with the Berlin survey, which is lower than our value of 64.5% in patients over 508. Notably, recent evidence suggests that the STOPBANG survey has higher sensitivity and diagnostic odds ratios than both the Berlin and STOP surveys, and recommends its use in the absence of available PSG11. These differences may also be explained by differences in catchment area: our catchment area is largely rural whereas above studies catchment areas are primarily urban.
Previous studies have shown that of hospitalized patients with positive STOPBANG screenings, approximately 2/3 will have a form of sleep-disordered breathing12. The identification, prompt diagnosis and treatment of individuals at risk for OSA could greatly benefit patients and healthcare systems alike. A significant barrier to increasing identification and treatment is awareness, as undiagnosed individuals are more likely to have lower functional health literacy and less knowledge of OSA’s symptoms and complications13,14. Further, previous work has shown that educational programs can improve CPAP adherence after diagnosis by 52%,15 though evidence that educational programs impact on pursual of follow-up PSG has not been investigated.
This study finds that patients who snore and are younger may be more likely to consider undergoing PSG to rule in or out OSA. One explanation for this finding may relate to patients’ health literacy. For example, if patients’ preexisting conception of risk factors for OSA centers around snoring, then they may be more likely to endorse follow up if they report snoring. Snoring has been shown to be the most readily identified symptom of OSA compared to apnea and EDS (6.5%, 4.7% and 1.2%, respectively) in a randomly selected population in Singapore13. Additionally, previous work comparing patients that self-reported OSA and newly diagnosed patients from a random screening found an inverse association between snoring and previous diagnosis of OSA – indicating that those previously diagnosed were more likely to be snorers14. Our finding that patients that report snoring are more willing to consider follow-up testing may in part explain this phenomenon. Thus, while it is important to promote continued awareness of snoring as a symptom of OSA, raising awareness of the other risk factors such as choking and EDS may increase willingness to undergo screening. Further work should clarify what symptoms patient’s perceive are associated with OSA in order to identify areas for targeted interventions.
A similar idea could explain the differences in age and interest in follow-up that we observed. It is possible that as health literacy continues to improve and the younger population gains better access to information through increased reliance on the internet, knowledge around the impacts of OSA on QOL may be rising. However, other evidence suggests that older populations tend to be more knowledgeable about OSA, and would argue against this hypothesis13. An alternative explanation may relate to the state of health of the patients on our wards. In general, younger patients on the floors are less complex and have longer life expectancies and may see treating OSA as more impactful on their long-term health.
Limitations
Our findings must be interpreted with the following limitations. Firstly, this study is limited by the methodology of the STOPBANG survey. Because much of the survey is reliant on patient-reported data, patients with language barriers and those lacking capacity were unable to participate. Further, the STOPBANG survey is by no means diagnostic; follow-up PSG is the standard for diagnosis of OSA. While this study investigated the relationship between demographic factors and patient interest in completing a PSG, we have no data confirming OSA via PSG or home respiratory polygraphy. Additionally, our sample size of 154 patients is notably smaller than other studies by more than an order of magnitude9,12. Secondly, as this study was conducted at a tertiary care center that serves a large rural catchment area, the patients surveyed may differ from patients in community hospitals or non-referral centers. In contrast, the major strength of this study is the investigation of all patients regardless of perceived risk, which enables us to extrapolate the prevalence of OSA risk in inpatient settings without limiting to subsets of patients.
Conclusion
Taken together, our results confirm the high prevalence of diagnosed OSA (17.9%) and patients with high OSA risk (57.8%) in inpatient populations. The finding that younger patients are 10 times more likely to consider PSG support arguments for early screening for OSA. Additionally, the finding that snorers are 3 times more likely to consider PSG may reflect the narrow focus of public awareness of OSA. Emphasizing early screening and awareness of the various symptoms associated with OSA may be strategies to alleviate the financial and QOL burden that undiagnosed OSA places on health systems and patients.
Author Information
Corresponding Author
Matthew Pelton
mpelton1@pennstatehealth.psu.edu
Author Contributions
All authors have given approval to the final version of the manuscript.
Funding Sources
The authors received no financial support for the research, authorship, and/or publication of this article.
Disclosures
No authors have any disclosures or conflicts of interest at this time.
References
1. Gallup. (2005). Omnibus Sleep in America Poll. (pp. 1-51): National Sleep Foundation.
2. Watson, N. F. (2016). Health Care Savings: The Economic Value of Diagnostic and Therapeutic Care for Obstructive Sleep Apnea. J Clin Sleep Med, 12(8), 1075-1077.
3. Marin, J. M., Carrizo, S. J., Vicente, E., & Agusti, A. G. (2005). Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet, 365(9464), 1046-1053.; Campos-Rodriguez, F., Martinez-Garcia, M. A., de la Cruz-Moron, I., Almeida-Gonzalez, C., Catalan-Serra, P., & Montserrat, J. M. (2012). Cardiovascular mortality in women with obstructive sleep apnea with or without continuous positive airway pressure treatment: a cohort study. Ann Intern Med, 156(2), 115-122.
4. Chung, F., Subramanyam, R., Liao, P., Sasaki, E., Shapiro, C., & Sun, Y. (2012). High STOP-Bang score indicates a high probability of obstructive sleep apnoea. Br J Anaesth, 108(5), 768-775. doi: 10.1093/bja/aes022; Chung, F. (2012). STOPbang.ca.
5. Chung, F., Yegneswaran, B., Liao, P., Chung, S. A., Vairavanathan, S., Islam, S., Shapiro, C. M. (2008). STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology, 108(5), 812-821.
6. Kumar, S., McElligott, D., Goyal, A., Baugh, M., & Ionita, R. N. (2010). Risk of Obstructive Sleep Apnea (OSA) in Hospitalized Patients. CHEST, 138(4), 779A.
7. Sharma, S., Mather, P. J., Efird, J. T., Kahn, D., Shiue, K. Y., Cheema, M., Quan, S. F. (2015). Obstructive Sleep Apnea in Obese Hospitalized Patients: A Single Center Experience. J Clin Sleep Med, 11(7), 717-723.
8. Shear, T. C., Balachandran, J. S., Mokhlesi, B., Spampinato, L. M., Knutson, K. L., Meltzer, D. O., & Arora, V. M. (2014). Risk of sleep apnea in hospitalized older patients. J Clin Sleep Med, 10(10), 1061-1066.
9. Finkel, K. J., Searleman, A. C., Tymkew, H., Tanaka, C. Y., Saager, L., Safer-Zadeh, E., Avidan, M. S. (2009). Prevalence of undiagnosed obstructive sleep apnea among adult surgical patients in an academic medical center. Sleep Med, 10(7), 753-758.
10. Sharma, S., Mather Paul, J., Efird Jimmy, T., Kahn, D., Shiue Kristin, Y., Cheema, M., . . . Quan Stuart, F. Obstructive Sleep Apnea in Obese Hospitalized Patients: A Single Center Experience. Journal of Clinical Sleep Medicine, 11(07), 717-723.
11. Chiu, H. Y., Chen, P. Y., Chuang, L. P., Chen, N. H., Tu, Y. K., Hsieh, Y. J., Guilleminault, C. (2017). Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: A bivariate meta-analysis. Sleep Med Rev, 36, 57-70.
12. Sharma, S., Chowdhury, A., Tang, L., Willes, L., Glynn, B., & Quan, S. F. (2016). Hospitalized Patients at High Risk for Obstructive Sleep Apnea Have More Rapid Response System Events and Intervention Is Associated with Reduced Events. PLoS One, 11(5), e0153790.
13. Sia, C.-H., Hong, Y., Tan, L. W. L., van Dam, R. M., Lee, C.-H., & Tan, A. (2017). Awareness and knowledge of obstructive sleep apnea among the general population. Sleep Med, 36, 10-17.
14. Li, J. J., Appleton, S. L., Wittert, G. A., Vakulin, A., McEvoy, R. D., Antic, N. A., & Adams, R. J. (2014). The relationship between functional health literacy and obstructive sleep apnea and its related risk factors and comorbidities in a population cohort of men. Sleep, 37(3), 571-578.
15. Willemin, M. C., Fry, S., Peres, S., Wallaert, B., & Mallart, A. (2013). Effects of an educational program in non-adherent apneic patients treated with continuous positive airway pressure. Rev Pneumol Clin, 69(2), 70-75.