Introduction
Hyperglycemia in the perioperative period is associated with higher rates of adverse events in patients with and without diabetes mellitus.1 Specifically, in patients with diabetes, hyperglycemia correlates with delayed wound healing as well as increases in surgical site infection frequency, length of hospital stay, incidence of postoperative pneumonia, incidence of myocardial infarction, and mortality rates.2-4 In contrast, strict blood glucose control in diabetic patients is associated with an increased risk of hypoglycemia.5 There is varying evidence to support the association of strict glycemic control with the prevention of surgical site infections,6 stroke, or death.5
Prior research regarding standardized diabetes medication instruction programs has yielded mixed results in optimizing perioperative plasma glucose levels. DiNardo et al., Franco et al., and Vongsumran et al. achieved decreased rates of postoperative hyperglycemia in patients after a standardized glucose management intervention.7-9 However, Cuevas et al. found no significant changes in plasma glucose levels with a standardized instruction program.10 Additionally, Vongsumran et al. found an association between standardized preoperative instruction and lower incidences of postoperative complications, such as intensive care unit admissions and acute kidney injury.9 Even though several studies describe standardized instruction programs and glycemic control, there is limited data on the relationship between the standardization of preoperative diabetic medications and rates of surgical complications.
The Anesthesia Preoperative Center (APEC) at Penn State Hershey Medical Center does not have a standardized protocol for preoperative management of diabetic medications. Current practices were designed and implemented by individual providers based on patient circumstances, leading to wide variability in management. In January 2019, APEC implemented the standardization of preoperative diabetic medication instruction, for both oral hypoglycemics and insulin medication regimens. This was created to systemically optimize glucose levels for elective non-cardiac surgery and reduce complications in the perioperative period. We hypothesized that there would be an improvement in postoperative glucose control as well as a decrease in postoperative complications after January 2019. To test the latter, we tracked diabetic ketoacidosis (DKA) and hyperosmolar hyperglycemic syndrome (HHS), surgical site infection rates, postoperative infection rates, returning to the operating room, and 30-day readmission rates after the implementation of standardization.
Methods
The Standardized Instruction Program
The standardized preoperative diabetic medication instructions were developed according to several recommendations regarding perioperative management of diabetes mellitus.4,11 The protocol contains instructions for injectables (long-acting, intermediate-acting and mixes, short-acting, and non-insulin injectables) and oral medications (secretagogues, dipeptidyl peptidase-4 [DPP-4] inhibitors, sodium-glucose cotransporter [SGLT-2] inhibitors, thiazolidinediones, biguanides, and glucagon-like peptide-1 [GLP-1] agonists). Instructions were provided to all patients specifying which medications to take, which medications to hold, and what doses should be administered the day before and the day of surgery (Table 1). Patients who used long-acting insulin were instructed to take their usual dose the morning of the day before surgery, 80% of the usual dose (or 50% of the usual dose if blood glucose was less than 100 mg/dL) the night before surgery, and 80% of the usual dose the day of surgery. Patients who use intermediate-acting insulin and mixes were asked to take their usual dose the day before surgery and 50% of the usual dose of insulin the day of surgery. However, if the blood glucose was less than 100 mg/dL, they were instructed to hold the dose. Patients who took short-acting insulin, non-insulin injectables, secretagogues, biguanides, and GLP-1 agonists were recommended to take their usual dose the day before surgery and hold the dose the day of surgery. Patients were instructed to hold SGLT-2 inhibitors four days prior to surgery and on the day of surgery. Patients were instructed to take DDP-4 inhibitors and thiazolidinediones the day before and the day of surgery. Patients that took weekly medications, were instructed to take the medication prior to surgery. Patients were asked on the day of surgery if they adhered to the standardized medication protocol.
Table 1. Standardized preoperative diabetic medication instruction provided to patients after January 1, 2019.
|
Medication |
Day before surgery |
Day of surgery |
|
|
Long-acting insulin |
AM Dose |
Usual dose |
80% of usual dose |
|
PM Dose |
80% of usual dose If BG<100 mg/dL = 50% usual dose* |
||
|
Intermediate-acting and mixes |
AM Dose |
Usual dose |
50% of usual dose If BG<100 mg/dL = Hold |
|
PM Dose |
Usual dose |
||
|
Short-acting insulin |
AM Dose |
Usual dose |
Hold |
|
PM Dose |
Usual dose |
||
|
Non-insulin injectables |
AM Dose |
Usual dose |
Hold |
|
PM Dose |
Usual dose |
||
|
Secretagogues |
Take |
Hold |
|
|
DDP-4 inhibitors |
Take |
Take |
|
|
Sodium-glucose cotransporter inhibitors (SGLT-2) |
Hold for 4 days prior to surgery |
Hold |
|
|
Thiazolidinediones |
Take |
Take |
|
|
Biguanides |
Take |
Hold |
|
|
Glucagon-like peptide (GLP-1) agonists |
Take |
Hold |
|
*BG checked prior to night-time dose of long-acting insulin – if BG<100 mg/dL, give 50% of usual dose. Abbreviation: BG, blood glucose.
Study Design
Patients were allocated into two groups: 1) the pre-intervention group (control group) included patients seen by the APEC clinic between January 2018 and January 2019 who did not receive standardized diabetic medication instruction, and 2) the post-intervention group included patients that were seen after the implementation of standardized diabetic medication instruction from January 2019 to January 2020.
Patients were included if they were over 18 years old, had a diagnosis of diabetes mellitus type 1 or 2, were using insulin therapy or oral hypoglycemic medications, were not pregnant, were evaluated in the APEC clinic 30 days before surgery, or underwent elective non-cardiac surgery in the timeframe listed above. Patients were excluded from analysis if they received oral steroid therapy three months prior to surgery, underwent urgent or emergent procedures, underwent cardiac surgery, or received dextrose-containing intravenous fluid (including antibiotic dilutant) intra-operatively.
Preoperative glucose levels were measured in the preoperative unit on the day of surgery. Post-operative glucose levels were measured while the patient was in the post-operative care unit. There was no standardized time before or after the operation in which these parameters were measured.
Data Collection
Study data were collected and managed using Research Electronic Data Capture (REDCap) electronic data capture tools hosted at Penn State Health Milton S. Hershey Medical Center and Penn State College of Medicine. REDCap is a secure, web-based software platform designed to support data capture for research studies and provides the following: 1) an intuitive interface for validated data capture, 2) audit trails for tracking data manipulation and export procedures, 3) automated export procedures for seamless data downloads to common statistical packages, and 4) procedures for data integration and interoperability with external sources.12
This study was sent to the Pennsylvania State University College of Medicine Institutional Review Board for review and was approved.
Analysis
All statistical analyses were performed using the SAS statistical package (version 9.4; SAS Institute, Inc., Cary, NC, USA). Continuous variables are presented as mean ±SD, and unadjusted analyses were conducted using two-sample t-tests. Categorical variables are presented as counts and percentages, and unadjusted analyses were conducted using chi-square tests.
Multivariable logistic regression was used to compare secondary outcomes between groups while adjusting for covariates (age, sex, BMI, hypertension, comorbidities, APEC instructions followed, anesthesia type, intraoperative adrenergic drug). Analysis of covariance was used to evaluate differences between postoperative glucose measurements and time to discharge pre- and post-standardization while adjusting for covariates (age, sex, BMI, diabetes type, glycated hemoglobin [HbA1c], hypertension, comorbidities, American Society of Anesthesiology [ASA] status, preoperative infection, APEC instructions followed, anesthesia type, intraoperative steroid, intraoperative adrenergic drug). We determined if APEC instructions were followed by confirming medication education adherence with patients on the day of surgery. Due to low frequency, the following preoperative diagnoses were combined to form the preoperative comorbidities parameter: transient ischemic attack/cerebrovascular accident (TIA/CVA), coronary artery disease (CAD), congestive heart failure (CHF), chronic kidney disease/end-stage renal disease (CKD/ESRD), heart failure, myocardial infarction, respiratory failure, and pneumonia. Statistical significance was set at a P value of <0.05.
Results
The baseline characteristics of the patients are shown in Table 2. A total of 350 patients met the inclusion and exclusion criteria. Of these, 167 (47.7%) were in the pre-protocol group and 183 (52.3%) were in the post-protocol group. The average age was 58.8 ±16.6 years, the average BMI of 35.7 ±10.64 kg/m2, and the group consisted of 50.7% females. The majority (85.3%) of participants were previously diagnosed with type 2 diabetes with an average HbA1c of 7.9% ±1.82%. The majority of participants (81.3%) had an ASA status of 3, indicating a patient with a severe systemic disease that is not life-threatening. Apart from BMI, which was significantly increased in the post-standardization group, preoperative antibiotic use, which was more common in the pre-standardization group, and adherence to APEC instructions, which was more common in the post-standardization group, patient data did not demonstrate significant differences between the two groups.
Table 2. Baseline patient characteristics.
|
Pre-standardization group (n=167) |
Post-standardization group (n=183) |
Total (n=350) |
P value (for chi-square test or two-sample t-test) |
|
|
Demographic Information |
||||
|
Age (years), mean (SD) |
59.2 (15.72) |
58.4 (17.41) |
58.8 (16.60) |
0.66 |
|
Females, n (%) |
80 (47.9%) |
97 (53.3%) |
177 (50.7%) |
0.31 |
|
BMI (kg/m2), mean (SD) |
34.5 (8.94) |
36.8 (11.92) |
35.7 (10.64) |
0.04 |
|
Diabetes Type, n (%) |
0.89 |
|||
|
Type 1 |
24 (14.4%) |
27 (14.9%) |
51 (14.7%) |
|
|
Type 2 |
143 (85.6%) |
154 (85.1%) |
297 (85.3%) |
|
|
HbA1c, mean (SD) (%) |
8.0 (2.06) |
7.9 (1.57) |
7.9 (1.82) |
0.57 |
|
Hypertension, n (%) |
130 (77.8%) |
150 (82.9%) |
280 (80.5%) |
0.24 |
|
Any Comorbidities^, n (%) |
110 (65.9%) |
112 (61.9%) |
222 (63.8%) |
0.44 |
|
ASA Status, n (%) |
0.84 |
|||
|
2 |
14 (8.4%) |
15 (8.3%) |
29 (8.4%) |
|
|
3 |
134 (80.2%) |
148 (82.2%) |
282 (81.3%) |
|
|
>=4 |
19 (11.4%) |
17 (9.4%) |
36 (10.4%) |
|
|
Preoperative Information |
||||
|
Preoperative glucose (mg/dL), mean (SD) |
158.5 (63.22) |
154.3 (56.32) |
156.3 (59.61) |
0.52 |
|
Preoperative antibiotic use, n (%) |
29 (17.4%) |
18 (10.0%) |
47 (13.5%) |
0.045 |
|
Preoperative ongoing infection, n (%) |
8 (4.8%) |
14 (7.7%) |
22 (6.3%) |
0.26 |
|
APEC instructions followed, n (%) |
3 (1.8%) |
164 (90.6%) |
167 (48.0%) |
<0.001 |
|
Intraoperative Information |
||||
|
Type of anesthesia, n (%) |
0.52 |
|||
|
General |
108 (64.7%) |
121 (66.9%) |
229 (65.8%) |
|
|
MAC |
53 (31.7%) |
57 (31.5%) |
110 (31.6%) |
|
|
Neuraxial |
6 (3.6%) |
3 (1.7%) |
9 (2.6%) |
|
|
Intraoperative steroid use, n (%) |
33 (19.8%) |
46 (25.7%) |
79 (22.8%) |
0.19 |
|
Adrenergic drug use, n (%) |
75 (44.9%) |
46 (25.7%) |
121 (35.0%) |
<0.001 |
|
Postoperative Glucose (mg/dL), mean (SD) |
162.0 (57.92) |
169.8 (62.88) |
165.7 (60.30) |
0.36 |
^Comorbidities: chronic kidney disease/end-stage renal disease (CKD/ESRD), congestive heart failure (CHF), coronary artery disease (CAD), myocardial infarction, pneumonia, respiratory failure, and transient ischemic attack/cerebrovascular accident (TIA/CVA).
Additional abbreviations: APEC, Anesthesia Perioperative Evaluation Center; ASA, American Society of Anesthesiologists; BMI, body mass index; HbA1c, glycated hemoglobin; MAC, monitored anesthesia care.
With regard to the type of anesthesia administered, there was no statistically significant difference between the pre- and post-standardization groups. Except for intraoperative adrenergic drug use, which was more common in the pre-standardization group, there was no significant difference regarding intraoperative medications.
There was no significant difference between pre- and post-standardization protocols for postoperative glucose levels (162.0 mg/dL ±57.92 vs. 169.8 mg/dL ±60.30, P=0.36) (Figure 1). There was also no significant difference between pre- and post-standardization protocol in the secondary outcomes (Table 3) of time to discharge in days (4.79 ±0.95 vs. 4.55 0.94, P=0.73), postoperative surgical site infection (OR=13.765; 95% CI [0.40, 471.1]), postoperative infection (OR=0.62; 95% CI [0.22, 1.73]), diabetes complications such as DKA or HHS (OR=1.29; 95% CI [0.04, 43.08]), readmission to the hospital within 30-days (OR=1.15; 95% CI [0.40, 3.32]), and return to the operating room (OR=0.61; 95% CI [0.21, 1.73]).
Figure 1. Average postoperative blood glucose concentration in patients pre- and post-standardization of glucose optimization education program.
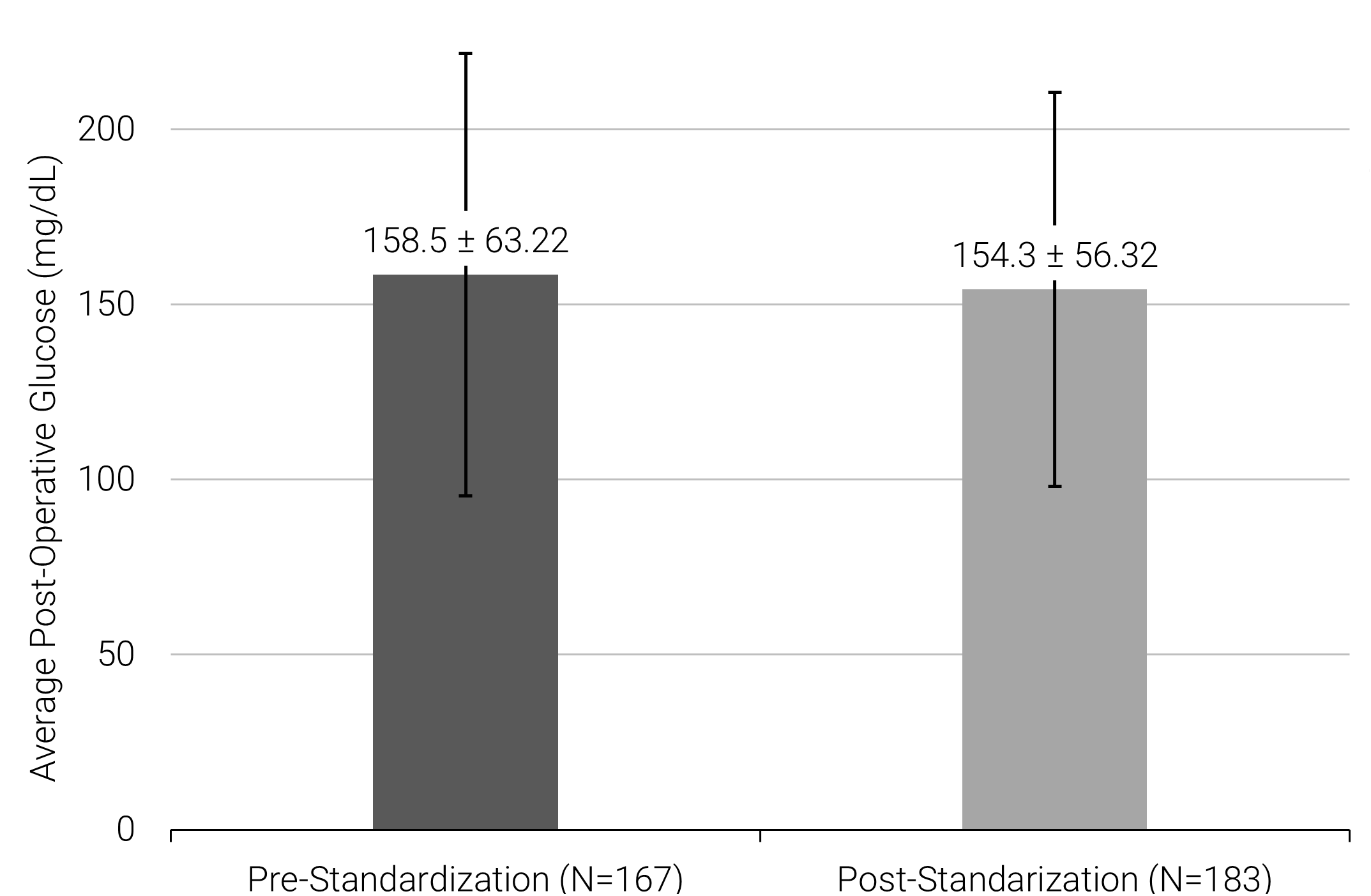
Abbreviations: DKA, diabetic ketoacidosis; DM, diabetes mellitus; HHS, hyperosmotic hyperglycemic syndrome; SSI, surgical site infection.
Table 3. Multivariable logistic regression for binary outcomes of postoperative complications.
|
Postoperative complication |
Odds ratios (95% CIs) |
P value |
|
Postoperative SSI |
13.77 (0.40, 471.1) |
0.15 |
|
Postoperative infection |
0.62 (0.22, 1.73) |
0.36 |
|
DM complication (DKA, HHS) |
1.29 (0.04, 43.08) |
0.89 |
|
Readmission within 30 days |
1.15 (0.40, 3.32) |
0.8 |
|
OR return |
0.61 (0.21, 1.73) |
0.35 |
Discussion
The purpose of this study was to evaluate the effectiveness of a standardized preoperative diabetic medication instruction program in diabetic patients undergoing elective noncardiac surgical procedures. In particular, postoperative blood glucose levels and surgical complication rates were measured to assess the success of the program.
Our results indicate that standardization of glucose optimization instruction does not demonstrate significant differences in average postoperative plasma glucose concentrations nor does it decrease adverse events in the perioperative period. This implies that the cause of postoperative surgical complications is unrelated to diabetes education in this population of patients. Similarly, Cuevas et al. found that the implementation of a standardized medication instruction program did not result in significant changes in plasma glucose levels.10 There were some studies that did find significant changes in plasma glucose levels with a standardization of medication education. Notably, Vongsumran et al. found a statistically significant decrease in plasma glucose levels, however there was no statistical difference in clinical outcome or complications as a result of this decrease in plasma glucose level in the postoperative period.9
Prior literature has stated that preoperative blood glucose values were the most important predictor of perioperative blood glucose control.7 The current recommendations are maintaining a blood glucose level of less than 180 mg/dL in the perioperative state, as there are increased incidences of adverse events and poorer outcomes in patients with poor glucose control.1,13 In our study, the average pre- and postoperative blood glucose levels in both groups were within the recommendation, suggesting that our patients had adequate glycemic control. This may be a reason for the lack of significant results after the implementation of the program.
Even though our patients demonstrated adequate short-term glycemic control in the perioperative period, both groups had elevated HbA1c levels. Although HbA1c is a tool that measures a patient’s average glucose levels over a 3-month period, elevated HbA1c values have not been found to be associated with an increased risk of postoperative infection or surgical complications.14,15 This suggests that elevated HbA1c levels are less prognostic in short-term surgical complication rates.
Although this program did not support our hypotheses that a standardized perioperative glucose optimization program reduces postoperative hyperglycemia and postoperative complication rates, it did provide insight into future studies that could be performed on the topic. An overwhelming majority of our patients reported following the APEC medication instructions and therefore received consistent evidence-based instruction. Studies that implemented similar programs demonstrated increased provider knowledge and confidence in having a correct answer to a knowledge-based question.10 Additionally, a standardized diabetic medication education program increased the efficiency of providing preoperative education and improved patient understanding and satisfaction.10 Future studies are needed to investigate how a standardized perioperative glucose optimization program affects provider and patient interactions.
Limitations
While the data provides insight into the use of a standardized perioperative glucose optimization program at a large academic facility, there are certain limitations to the study. The similarities between groups may be attributed to the overall low frequency of assessed outcomes. This study demonstrated that these adverse events are rare and subsequent analysis requires a larger sample size. The lack of significant results in this study may be attributed to patient selection, as the current selection criteria include patients who are on oral hypoglycemics and/or insulin. Further research may be conducted to evaluate the specific effects of the standardization of individual medications.
In addition, the lack of significant results may be due to the exclusive analysis of elective non-cardiac surgeries in our study. Elective surgeries may be canceled at the discretion of the anesthesiologist and the surgeon in the event of severe preoperative hyperglycemic measurements. We have not collected data regarding the number of canceled surgeries before and after the implementation of the standardized education instruction. Finally, there was no established timeframe for preoperative and postoperative glucose level collection. There is likely variability between patients in the time between collection and the start and end of the operation.
Conclusion
Glycemic management is of the utmost importance in perioperative management of diabetes. Although our implementation of a standardized diabetic medication education program did not result in significant changes in postoperative blood glucose levels or postoperative complications, our program found high rates of adherence once implemented. Further research with a larger population is warranted to assess the impact of a standardized medication protocol on postoperative complications in diabetic patients following noncardiac elective surgery.
Author Information
Corresponding Author
Olivia C. Calisi, B.A.
ocalisi@pennstatehealth.psu.edu
Author Contributions
All authors have given approval to the final version of the manuscript.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Disclosures
No authors have any disclosures or conflicts of interest at this time.
Acknowledgements
None.
References
- Kotagal M, Symons RG, Hirsch IB, et al. Perioperative Hyperglycemia and Risk of Adverse Events Among Patients With and Without Diabetes. Ann Surg. 2015;261(1):97-103. doi:10.1097/SLA.0000000000000688
- Frisch A, Chandra P, Smiley D, et al. Prevalence and Clinical Outcome of Hyperglycemia in the Perioperative Period in Noncardiac Surgery. Diabetes Care. 2010;33(8):1783-1788. doi:10.2337/dc10-0304
- Stryker LS, Abdel MP, Morrey ME, Morrow MM, Kor DJ, Morrey BF. Elevated Postoperative Blood Glucose and Preoperative Hemoglobin A1C Are Associated with Increased Wound Complications Following Total Joint Arthroplasty. J Bone Jt Surg. 2013;95(9):808-814. doi:10.2106/JBJS.L.00494
- Duggan EW, Carlson K, Umpierrez GE. Perioperative Hyperglycemia Management. Anesthesiology. 2017;126(3):547-560. doi:10.1097/ALN.0000000000001515
- de Vries FEE, Gans SL, Solomkin JS, et al. Meta-analysis of lower perioperative blood glucose target levels for reduction of surgical-site infection. Br J Surg. 2017;104(2):e95-e105. doi:10.1002/bjs.10424
- Kao LS, Meeks D, Moyer VA, Lally KP. Peri-operative glycaemic control regimens for preventing surgical site infections in adults. Cochrane Database Syst Rev. Published online July 8, 2009. doi:10.1002/14651858.CD006806.pub2
- DiNardo M, Donihi AC, Forte P, Gieraltowski L, Korytkowski M. Standardized Glycemic Management and Perioperative Glycemic Outcomes in Patients with Diabetes Mellitus who Undergo Same-Day Surgery. Endocr Pract. 2011;17(3):404-411. doi:10.4158/EP10316.OR
- Franco T, Rupp S, Williams B, Blackmore C. Effectiveness of standardised preoperative assessment and patient instructions on admission blood glucose for patients with diabetes undergoing orthopaedic surgery at a tertiary referral hospital. BMJ Open Qual.2019;8(2):e000455. doi:10.1136/bmjoq-2018-000455
- Vongsumran N, Buranapin S, Manosroi W. Standardized Glycemic Management versus Conventional Glycemic Management and Postoperative Outcomes in Type 2 Diabetes Patients Undergoing Elective Surgery. Diabetes, Metab Syndr Obes Targets Ther. 2020;Volume 13:2593-2601. doi:10.2147/DMSO.S262444
- Cuevas DK, Rucker MT, Johnson DT, Crerar C, Wofford K, Bonds R. Implementation of a Standardized Preoperative Diabetes Medication Guideline and its Effect on Day of Procedure Blood Glucose Levels. J PeriAnesthesia Nurs. 2019;34(2):303-309. doi:10.1016/j.jopan.2018.05.013
- Sreedharan R, Abdelmalak B. Diabetes Mellitus. Anesthesiol Clin. 2018;36(4):581-597. doi:10.1016/j.anclin.2018.07.007
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi:10.1016/j.jbi.2008.08.010
- Sheehy AM, Gabbay RA. An Overview of Preoperative Glucose Evaluation, Management, and Perioperative Impact. J Diabetes Sci Technol. 2009;3(6):1261-1269. doi:10.1177/193229680900300605
- Latham R, Lancaster AD, Covington JF, Pirolo JS, Thomas CS. The Association of Diabetes and Glucose Control With Surgical-Site Infections Among Cardiothoracic Surgery Patients. Infect Control Hosp Epidemiol. 2001;22(10):607-612. doi:10.1086/501830
- Acott AA, Theus SA, Kim LT. Long-term glucose control and risk of perioperative complications. Am J Surg.2009;198(5):596-599. doi:10.1016/j.amjsurg.2009.07.015
