
Associated Outcomes of Tranexamic Acid Use in Non-surgical Chronic Subdural Hematomas
Andrea Lin, B.S.1; David R. Hallan, M.D.2; Elias Rizk, M.D.2
1The Pennsylvania State University College of Medicine, Hershey, PA;2Department of Neurosurgery, Penn State Health Milton S. Hershey Medical Center, Hershey, PA
KEYWORDS: Neurosurgery, chronic subdural hematoma, tranexamic acid, outcomes, mortality rate
Purpose: Tranexamic acid (TXA) is a compound used to treat many bleeding conditions by inhibiting plasmin activity via binding to plasminogen and reducing fibrinolysis and inflammation. The role of TXA in the non-surgical management of chronic subdural hematomas (cSDH) has been studied previously, but data has been controversial. Mixed reports show TXA can reduce hematoma volume or result in complete resolution, while others show no benefit in reducing cSDH recurrence. Therefore, examined the impact of TXA in patients with cSDH who do not undergo burrhole drainage or middle meningeal artery embolization, and determine whether outcomes are associated with other complications. Methods: We performed a retrospective case-control analysis using a multi-institutional database (TriNetX). We reviewed non-acute subdural hematoma patients who did not undergo surgical treatment and were treated with or without TXA. The primary endpoint was mortality at 6 months. Secondary endpoints included ventilator dependence, seizure, venous thromboembolism, myocardial infarction (MI), cerebral infarction, and percutaneous endoscopic gastrostomy (PEG). Cohorts were propensity score-matched for confounding variables. Results: 470 patients were identified from TXA (cohort 1) and non-TXA (cohort 2) populations. The mean age at cSDH was 57.5 vs. 59.3 years. Mortality was seen in 93 patients (19.79%) in cohort 1 vs 73 (15.53%) in cohort 2 (P=0.09), ventilator dependence was 4.26% vs 2.55% (P=0.15), PEG placement was 5.75% vs 2.13% (P=0.004), seizures were 14.04% vs 11.28%(P=0.20), and venous thromboembolism was 8.09% vs 3.83% (P=0.006). There were too few patients with stroke and myocardial infarction for meaningful analysis of those outcomes. Conclusion: TXA use in non-acute subdural hematomas is associated with an increased incidence of venous thromboembolism and PEG tube placement. In addition, TXA use was not found to benefit mortality 6 months post-operatively. It was not significantly associated with ventilator dependence or the occurrence of seizures, stroke, or myocardial infarction. Further research is needed to determine if hematoma characteristics, such as volume, may be related to outcomes seen in this study.
Tranexamic acid (TXA) has been used to treat a number of bleeding conditions, with mixed results.1-14 The role of TXA in the conservative (non-surgical) management of chronic subdural hematomas (cSDH) is controversial. Some reports have shown that TXA reduces hematoma volume,15 others complete resolution of hematoma,16,17 and others demonstrate no benefit in reducing cSDH recurrence.18 cSDH has a high incidence in those age 70 or older, and with the increasing age of the population and high rates of recurrence from traditional management of burrhole or mini-craniotomy evacuation, optimal management of this neurosurgical disease is an area of increasing interest. We sought to examine the impact of tranexamic in cSDH who do not undergo burrhole drainage or middle meningeal artery embolization, leveraging a multicenter research network with matched controls.
Methods
Study Design
This was a retrospective comparative case-control study. We used a de-identified database network (TriNetX) to retrospectively query via ICD-10 and current procedural terminology codes to evaluate all patients with a diagnosis of non-traumatic non-acute subdural hematoma without surgical evacuation or endovascular intervention and with subsequent TXA use within 6 months of initial cSDH diagnosis (cohort 1) versus no subsequent TXA use (cohort 2). Data came from 57 healthcare organizations (HCOs) spanning 6 countries. Data includes demographics, diagnoses, medications, laboratory values, genomics, and procedures. The identity of the HCOs and patients is not disclosed to comply with ethical guidelines and prevent re-identification. Due to the database’s federated nature, an IRB waiver was been granted and no further IRB approval was necessary for this study. The data is updated daily. Previous literature informed our use of this database and its validity, and the network’s exact details have been previously described.19-22 The diagnosis was based on International Classification of Disease (ICD-10) codes and procedural codes via Common Procedural Terminology (CPT) codes. The index date was set at the date of cSDH.
Variables
Medical information included age at index date, as well as sex, race, and comorbidities of hypertension, acute kidney injury, diabetes, ischemic heart disease, heart failure, atrial fibrillation, disorders of lipoprotein metabolism and other lipidemias, obesity, history of nicotine dependence, chronic respiratory disease, cirrhosis, alcohol abuse or dependence, and peripheral vascular disease, recorded up to the date of the index date. Medication information included blood thinners, dexamethasone, and atorvastatin were also included. Our primary endpoint was mortality at 6 months. Secondary endpoints included ventilator dependence, seizure, venous thromboembolism (VTE), myocardial infarction (MI), cerebral infarction, and percutaneous endoscopic gastrostomy (PEG).
Analysis
Analysis was performed using unmatched and propensity score-matched cohorts with the greedy-nearest neighbor algorithm and a caliper of 0.1 pooled standard deviations. Hazard ratios were calculated using R’s survival package v3.2-3 and validated by comparing the output to SAS version 9.4. Chi-square analysis was performed on categorical variables. Significance was defined as a P value less than 0.05.
Results
A total of 471 patients from cohort 1 were identified versus 91,983 from cohort 2. After propensity score matching, 470 patients were identified in each cohort. After matching, the age at index was 57.49+-26.49 years and 59.29+-25.24 years for cohorts 1 and 2, respectively. 60.21% of cohort 1 were male, versus 59.79% in cohort 2. 69.79% % of patients were white in both cohorts, 14.68% vs. 15.11% were black or African American, and 13.83% vs. 12.55% were of unknown race. Baseline demographics and characteristics are shown in Table 1.
Table 1. Baseline characteristics after propensity score matching.
|
Before Matching |
After Matching |
||||||
|
Code |
Diagnosis |
Cohort 1 n (%) |
Cohort 2 n (%) |
Std diff. |
Cohort 1 n (%) |
Cohort 2 n (%) |
Std diff. |
|
AI |
Age at Index (mean) |
57.38 |
59.88 |
- |
57.49 |
59.29 |
- |
|
2106-3 |
White |
329 (69.85) |
64,248 (70.97) |
0.02 |
328 (69.79) |
328 (69.79) |
0 |
|
M |
Male |
284 (60.30) |
53,228 (58.79) |
0.03 |
283 (60.21) |
281 (59.79) |
0.01 |
|
F |
Female |
187 (39.70) |
37,289 (41.19) |
0.03 |
187 (39.79) |
189 (40.21) |
0.01 |
|
2054-5 |
Black or African American |
69 (14.65) |
10,816 (11.95) |
0.08 |
69 (14.68) |
71 (15.11) |
0.01 |
|
2131-1 |
Unknown Race |
65 (13.80) |
13,263 (14.65) |
0.02 |
65 (13.83) |
59 (12.55) |
0.04 |
|
2028-9 |
Asian |
<10 (<2.12) |
1,628 (1.80) |
0.02 |
<10 (<2.13) |
<10 (<2.13) |
0 |
|
I10-I16 |
Hypertensive diseases |
270 (57.33) |
45,748 (50.53) |
0.14 |
269 (57.23) |
267 (56.81) |
0.01 |
|
N17-N19 |
Acute kidney failure and chronic kidney disease |
153 (32.48) |
16,313 (18.02) |
0.34 |
152 (32.34) |
154 (32.77) |
0.01 |
|
R40 |
Somnolence, stupor and coma |
145 (30.79) |
15,802 (17.45) |
0.32 |
144 (30.64) |
147 (31.28) |
0.01 |
|
E78 |
Disorders of lipoprotein metabolism and other lipidemias |
138 (29.30) |
28,132 (31.07) |
0.04 |
138 (29.36) |
143 (30.43) |
0.02 |
|
I20-I25 |
Ischemic heart diseases |
115 (24.42) |
19,223 (21.23) |
0.08 |
115 (24.47) |
118 (25.11) |
0.01 |
|
E08-E13 |
Diabetes mellitus |
107 (22.72) |
18,243 (20.15) |
0.06 |
107 (22.77) |
103 (21.92) |
0.02 |
|
I50 |
Heart failure |
107 (22.72) |
11,163 (12.33) |
0.28 |
106 (22.55) |
110 (23.40) |
0.02 |
|
R53 |
Malaise and fatigue |
107 (22.72) |
16,407 (18.12) |
0.11 |
106 (22.55) |
116 (24.68) |
0.05 |
|
J40-J47 |
Chronic lower respiratory diseases |
92 (19.53) |
13,979 (15.44) |
0.11 |
92 (19.57) |
88 (18.72) |
0.02 |
|
I48 |
Atrial fibrillation and flutter |
92 (19.53) |
14,154 (15.63) |
0.10 |
92 (19.57) |
80 (17.02) |
0.07 |
|
R13 |
Aphagia and dysphagia |
76 (16.14) |
9,375 (10.36) |
0.17 |
76 (16.17) |
80 (17.021) |
0.02 |
|
R63 |
Symptoms and signs concerning food and fluid intake |
73 (15.50) |
6,636 (7.33) |
0.26 |
72 (15.32) |
82 (17.45) |
0.06 |
|
E65-E68 |
Overweight, obesity, and other hyperalimentation |
70 (14.86) |
8,184 (9.04) |
0.18 |
70 (14.90) |
71 (15.11) |
0.01 |
|
Z87.891 |
Personal history of nicotine dependence |
67 (14.23) |
10,426 (11.52) |
0.08 |
67 (14.26) |
62 (13.19) |
0.03 |
|
F17 |
Nicotine dependence |
61 (12.95) |
11,130 (12.29) |
0.02 |
61 (12.98) |
64 (13.61) |
0.02 |
|
F10.1 |
Alcohol abuse |
33 (7.01) |
7,425 (8.20) |
0.05 |
33 (7.02) |
45 (9.57) |
0.09 |
|
I73 |
Other peripheral vascular diseases |
24 (5.10) |
4,772 (5.27) |
0.01 |
24 (5.11) |
30 (6.38) |
0.05 |
|
F10.2 |
Alcohol dependence |
23 (4.88) |
4,845 (5.35) |
0.02 |
23 (4.89) |
32 (6.81) |
0.08 |
|
K74 |
Fibrosis and cirrhosis of liver |
21 (4.46) |
2,034 (2.25) |
0.12 |
21 (4.47) |
25 (5.32) |
0.04 |
|
1191 |
Aspirin |
191 (40.55) |
22,534 (24.89) |
0.34 |
190 (40.43) |
204 (43.40) |
0.06 |
|
11289 |
Warfarin |
55 (11.68) |
7,234 (7.99) |
0.12 |
54 (11.49) |
55 (11.70) |
0.01 |
|
1364430 |
Apixaban |
16 (3.40) |
1,740 (1.92) |
0.09 |
16 (3.40) |
19 (4.04) |
0.03 |
|
1114195 |
Rivaroxaban |
13 (2.76) |
1,187 (1.31) |
0.10 |
13 (2.77) |
11 (2.34) |
0.03 |
Mortality was seen in 93 patients (19.79%) in cohort 1 vs 73 (15.53%) in cohort 2 (OR=1.34; 95% CI [0.96, 1.88]), ventilator dependence was 4.26% vs 2.55% (OR=1.70; 95% CI [0.82, 3.51]), PEG placement was 5.75% vs 2.13% (P≤0.004), seizures were 14.04% vs 11.28% (P=0.20), and venous thromboembolism was 8.09% vs 3.83% (P=0.006). There were too few patients with the outcomes of stroke and myocardial infarction for meaningful analysis. Figure 1 shows a Kaplan-Meier survival curve for outcome deceased to 180 days comparing cohorts 1 and 2. The hazard ratio was 1.27 (95% CI [0.94, 1.73]). Table 2 shows outcomes after propensity score matching.
Figure 1. Kaplan-Meier survival analysis for outcome: deceased.
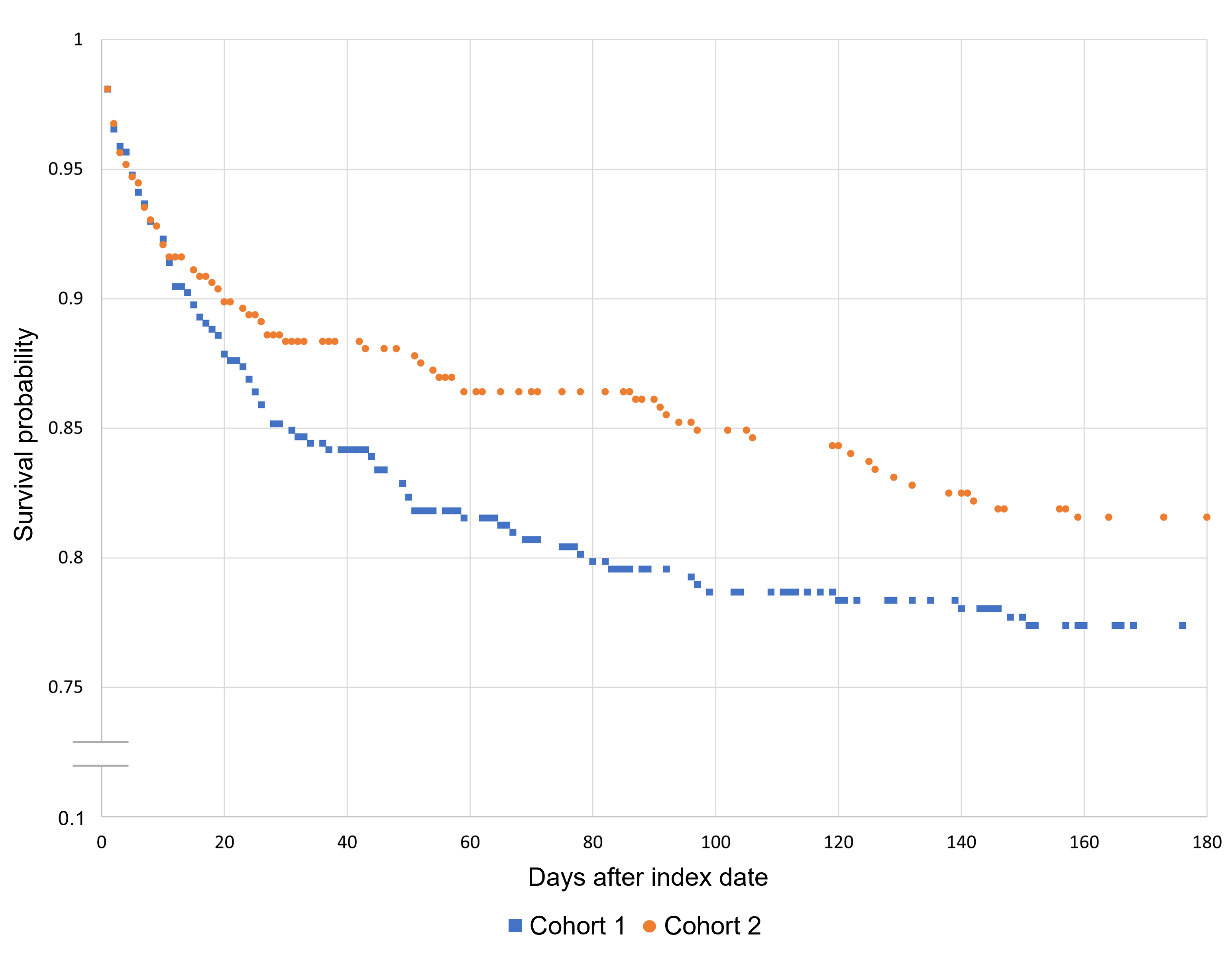
Table 2. Outcomes after propensity score matching.
|
Outcome |
Cohort 1 n (%) |
Cohort 2 n (%) |
Odds ratio (95% CI) |
P value |
|
Mortality |
93 (19.79) |
73 (15.53) |
1.34 (0.96, 1.88) |
0.09 |
|
Ventilator dependence |
20 (4.26) |
12 (2.55) |
1.70 (0.82, 3.51) |
0.15 |
|
PEG |
27 (5.75) |
≤ 10 (≤ 2.12) |
≥ 2.80 (1.34, 5.86) |
≤ 0.004 |
|
Seizures |
66 (14.04) |
53 (11.28) |
1.29 (0.87, 1.89) |
0.20 |
|
Venous thromboembolism |
38 (8.09) |
18 (3.83) |
2.21 (1.24, 3.93) |
0.06 |
Abbreviation: PEG, percutaneous endoscopic gastrostomy.
Discussion
cSDH is likely the result of many factors, including microbleeds, local inflammation, angiogenesis, and local coagulopathy.15 It is thought that the microbleeds may be the result of increased capillary permeability in the hematoma membrane,23 which is further induced by inflammation as the recruitment of inflammatory markers.24 These interleukins can enlarge gap junctions and thus cause increased permeability. It has been shown that cSDH fluid contains higher levels of interleukins and tumor necrosis factor-alpha than other fluids and that cSDH membranes are infiltrated with eosinophils and lymphocytes. The hematoma itself has been found to have increased eosinophil counts, which are thought to release plasminogen and cause hyperfibrinolysis. Through the COX-2 pathway, inflammation likewise stimulates overexpression of vascular endothelial growth factor, which can cause vascular leakage and cause the formation of new and often unstable capillaries that can further bleed and lead to hematoma formation.24 TXA is thought to decrease plasmin activity by reversibly binding to lysine sites on plasminogen, thereby reducing both fibrinolysis and inflammation. Theoretically, TXA is thus thought to break the cycle of new membrane formation, re-hemorrhage, increased vascular permeability, and inflammation, which will help the cSDH resorb over time and lead to clinical improvement.17 Contraindications to TXA use would be conditions that require fibrinolyses, such as a history of deep venous thrombosis, pulmonary embolism, ischemic stroke, patients who require anticoagulant therapy, or malignancy.25 Renal dysfunction would be another contraindication.25 It is also thought that TXA is associated with a dose-dependent increase in seizure risk.26
However, despite the theoretical benefits, our results demonstrate no significant mortality benefit in using TXA in cSDH. Furthermore, our results reveal an association between TXA use and increased incidence of VTE and PEG placement, the latter indicating a functional decline. On the other hand, there was no association between TXA and increased seizures.
In 2020, Wan et al. published a prospective, 3-center randomized trial looking at the effects of TXA for 21 days in reducing post-operative recurrence of cSDH, with outcomes measured at 6, 12, and 24 weeks. They found 10.2% recurrence (cSDH requiring reoperation) in the control group versus 4.8% recurrence in the TXA arm, but the result was not statistically significant (P=0.22).1 patient in the TXA group did suffer from cerebral infarction. There was no significant difference in residual hematoma volume after 6 weeks.18 This is in contrast to Tanweer et al.’s retrospective analysis in 2016 of 14 patients with 20 subdurals, who found that volume of cSDH was significantly (91.31% vs. 40.74%; P<0.001) reduced after TXA was started following subdural evacuation port system placement and removal, and no incidence of VTE was noted.27 Yamada et al. in 2020 performed a prospective study of 193 patients with cSDH treated with burrholes and then given TXA, goreisan, or no further intervention. They found no difference in the recurrence rate of cSDH but did find that the mean hematoma volume was reduced in the TXA group at 1, 2, and 3 months out. They also did not note any adverse events and concluded that TXA can safely reduce hematoma volume but does not affect recurrence.15 Kageyama et al. in 2013 found no recurrence or progression in 21 patients with cSDH treated with TXA for a median duration of 58 days. They concluded that cSDH could be treated with TXA without surgery.16 Kutty et al. in 2020 found similarly that in their cohort of 27 patients with cSDH treated with TXA resolution of cSDH, concluding that, in patients who do not show life-threatening symptoms, TXA is both safe and effective as an alternative treatment to surgery.17 There are ongoing randomized, controlled trials to investigate this issue further.28-30
Limitations
Our analysis was not without limitations. The major limitation of this study was that it was retrospective. Furthermore, due to the nature of the database, we were unable to collect patient-level data on specific outcomes. Patient-specific neurological deficits and any reasons for non-surgical management are not known. We were also unable to report radiology information, including hematoma volume. Additionally, we do not have information on the type of diagnostic test used for the confirmation of the disease, and some misclassification is inevitable.
Conclusion
TXA use in non-traumatic, non-acute subdural hematomas that were not surgically evacuated, is associated with an increased incidence of venous thromboembolism and PEG tube placement. In addition, TXA use was not found to reduce mortality, ventilator dependence, seizures, stroke, or myocardial infarction. While there exists some literature showing that TXA use does help decrease the size of chronic subdural hematomas, the decision to start TXA in cSDH should be made with the above associations taken into consideration.
Corresponding Author
Andrea Lin, B.S.
alin4@pennstatehealth.psu.edu
Author Contributions
All authors have given approval to the final version of the manuscript.
Funding Sources
The authors received no financial support for the research, authorship, and/or publication of this article.
Disclosures
No authors have any disclosures or conflicts of interest at this time.
Acknowledgements
None.

Penn State Journal of Medicine
Volume 3, 2022
ISSN: 2689-7350
DOI: 10.26209/psjm63078